Abstract
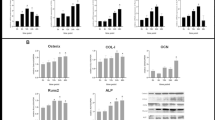
The study aims to explore the role of the ERK signaling pathway in the crosstalk between Dkk-1 and TNF-α in MC3T3E1 pre-osteoblasts under cyclic tensile/compressive stress. A forced four-point bending system was used to apply cyclic uniaxial tensile/compressive strain (2000 μ, 0.5 Hz) to MC3T3E1 cells. Dkk-1 and TNF-α expression were upregulated in MC3T3E1 cells under compressive strain. Cell proliferation, the cell cycle, osteogenesis-related gene (Wnt5a, Runx2, Osterix) expression, β-catenin expression, and the p-ERK/ERK ratio were significantly enhanced, whereas apoptosis, the RANKL/OPG ratio, and TNF-α expression were significantly attenuated, by Dkk-1 silencing. Dkk-1 expression increased and the effects of Dkk-1 silencing were reversed when exogenous TNF-α was added. Mechanically, TNF-α crosstalked with Dkk-1 through ERK signaling in MC3T3E1 cells. ERK signaling blockade impaired Dkk-1-induced TNF-α expression and TNF-α-mediated Dkk-1 expression. Dkk-1 and TNF-α crosstalked, partially through ERK signaling, in MC3T3E1 cells under compressive/tensile strain, synergistically modulating various biological behaviors of the cells. These findings not only provide mechanical insight into the cellular events and molecular regulation of orthodontic tooth movement (OTM), but also aid the development of novel strategies to accelerate OTM.






Similar content being viewed by others
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OTM:
-
Orthodontic tooth movement
- Dkk-1:
-
Dickkopf-related protein 1
- RANKL:
-
Nuclear factor-κB ligand
- OPG:
-
Osteoprotegerin
- TNF:
-
Tumor necrosis factor
- ERK:
-
Extracellular-regulated protein kinase
- MSCs:
-
Mesenchymal stem cells
- ATCC:
-
American Typical Culture Collection
- α-MEM:
-
α -Modified Eagle’s medium (
- FBS:
-
Fetal bovine serum
- siRNAs:
-
Small interfering RNAs
- RT-PCR:
-
Reverse-transcription polymerase chain reaction
- FITC:
-
Fluorescein isothiocyanate
- DAPI:
-
4′,6-Diamidino-2-phenylindole
References
Li Y, Jacox LA, Little SH, Ko CC (2018) Orthodontic tooth movement: the biology and clinical implications. Kaohsiung J Med Sci 34:207–214. https://doi.org/10.1016/j.kjms.2018.01.007
Huang H, Williams RC, Kyrkanides S (2014) Accelerated orthodontic tooth movement: molecular mechanisms. Am J Orthod Dentofac Orthop 146:620–632. https://doi.org/10.1016/j.ajodo.2014.07.007
Yamaguchi M, Fukasawa S (2021) Is inflammation a friend or foe for orthodontic treatment?: inflammation in orthodontically induced inflammatory root resorption and accelerating tooth movement. Int J Mol Sci. https://doi.org/10.3390/ijms22052388
Gkantidis N, Christou P, Topouzelis N (2010) The orthodontic-periodontic interrelationship in integrated treatment challenges: a systematic review. J Oral Rehabilit 37:377–390. https://doi.org/10.1111/j.1365-2842.2010.02068.x
Gyawali R, Bhattarai B (2017) Orthodontic management in aggressive periodontitis. Int Sch Res Notices 2017:8098154. https://doi.org/10.1155/2017/8098154
Jeon HH, Teixeira H, Tsai A (2021) Mechanistic insight into orthodontic tooth movement based on animal studies: a critical review. J Clin Med. https://doi.org/10.3390/jcm10081733
Li Y, Zhan Q, Bao M, Yi J, Li Y (2021) Biomechanical and biological responses of periodontium in orthodontic tooth movement: up-date in a new decade. Int J Oral Sci 13:20. https://doi.org/10.1038/s41368-021-00125-5
Kobayashi Y, Uehara S, Udagawa N, Takahashi N (2016) Regulation of bone metabolism by Wnt signals. J Biochem 159:387–392. https://doi.org/10.1093/jb/mvv124
Huang Y, Liu L, Liu A (2018) Dickkopf-1: current knowledge and related diseases. Life Sci 209:249–254. https://doi.org/10.1016/j.lfs.2018.08.019
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33:747–783. https://doi.org/10.1210/er.2011-1060
Lu J, Duan Y, Zhang M, Wu M, Wang Y (2016) Expression of Wnt3a, Wnt10b, β-catenin and DKK1 in periodontium during orthodontic tooth movement in rats. Acta Odontol Scand 74:217–223. https://doi.org/10.3109/00016357.2015.1090011
Han XL, Liu M, Voisey A, Ren YS, Kurimoto P, Gao T et al (2011) Post-natal effect of overexpressed DKK1 on mandibular molar formation. J Dent Res 90:1312–1317. https://doi.org/10.1177/0022034511421926
Chae WJ, Bothwell ALM (2019) Dickkopf1: an immunomodulatory ligand and Wnt antagonist in pathological inflammation. Differentiation 108:33–39. https://doi.org/10.1016/j.diff.2019.05.003
Sieper J (2011) Spondyloarthropathies in 2010: new insights into therapy-TNF blockade and beyond. Nat Rev Rheumatol 7:78–80. https://doi.org/10.1038/nrrheum.2010.224
Ma Y, Zhang X, Wang M, Xia Q, Yang J, Wu M et al (2018) The serum level of Dickkopf-1 in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int Immunopharmacol 59:227–232. https://doi.org/10.1016/j.intimp.2018.04.019
Wu M, Chen M, Ma Y, Yang J, Han R, Yuan Y et al (2018) Dickkopf-1 in ankylosing spondylitis: review and meta-analysis. Clin Chim Acta 481:177–183. https://doi.org/10.1016/j.cca.2018.03.010
Miranda TS, Napimoga MH, Feres M, Marins LM, da Cruz DF, da Silva HDP et al (2018) Antagonists of Wnt/β-catenin signalling in the periodontitis associated with type 2 diabetes and smoking. J Clin Periodontol 45:293–302. https://doi.org/10.1111/jcpe.12854
Katz S, Boland R, Santillán G (2006) Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in osteoblasts: involvement of mechanical stress-activated calcium influx, PKC and Src activation. Int J Biochem Cell Biol 38:2082–2091. https://doi.org/10.1016/j.biocel.2006.05.018
Wu Y, Zhang X, Zhang P, Fang B, Jiang L (2012) Intermittent traction stretch promotes the osteoblastic differentiation of bone mesenchymal stem cells by the ERK1/2-activated Cbfa1 pathway. Connect Tissue Res 53:451–459. https://doi.org/10.3109/03008207.2012.702815
Wang B, Du T, Wang Y, Yang C, Zhang S, Cao X (2011) Focal adhesion kinase signaling pathway is involved in mechanotransduction in MG-63 cells. Biochem Biophys Res Commun 410:671–676. https://doi.org/10.1016/j.bbrc.2011.06.054
Yan YX, Gong YW, Guo Y, Lv Q, Guo C, Zhuang Y et al (2012) Mechanical strain regulates osteoblast proliferation through integrin-mediated ERK activation. PLoS ONE 7:e35709. https://doi.org/10.1371/journal.pone.0035709
Pelaez D, Arita N, Cheung HS (2012) Extracellular signal-regulated kinase (ERK) dictates osteogenic and/or chondrogenic lineage commitment of mesenchymal stem cells under dynamic compression. Biochem Biophys Res Commun 417:1286–1291. https://doi.org/10.1016/j.bbrc.2011.12.131
Bougault C, Aubert-Foucher E, Paumier A, Perrier-Groult E, Huot L, Hot D et al (2012) Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS ONE 7:e36964. https://doi.org/10.1371/journal.pone.0036964
Sabio G, Davis RJ (2014) TNF and MAP kinase signalling pathways. Semin Immunol 26:237–245. https://doi.org/10.1016/j.smim.2014.02.009
Trotta R, Kanakaraj P, Perussia B (1996) Fc gamma R-dependent mitogen-activated protein kinase activation in leukocytes: a common signal transduction event necessary for expression of TNF-alpha and early activation genes. J Exp Med 184:1027–1035. https://doi.org/10.1084/jem.184.3.1027
Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH et al (2000) A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol 20:6084–6094. https://doi.org/10.1128/mcb.20.16.6084-6094.2000
Zheng L, Hu F, Bian W, Li Y, Zhang L, Shi L et al (2021) Dickkopf-1 perpetuated synovial fibroblast activation and synovial angiogenesis in rheumatoid arthritis. Clin Rheumatol 40:4279–4288. https://doi.org/10.1007/s10067-021-05766-9
Baloul SS (2016) Osteoclastogenesis and osteogenesis during tooth movement. Front Oral Biol 18:75–79. https://doi.org/10.1159/000351901
Wang Y, Wang J, Bai D, Song J, Ye R, Zhao Z et al (2013) Cell proliferation is promoted by compressive stress during early stage of chondrogenic differentiation of rat BMSCs. J Cell Physiol 228:1935–1942. https://doi.org/10.1002/jcp.24359
Lv PY, Gao PF, Tian GJ, Yang YY, Mo FF, Wang ZH et al (2020) Osteocyte-derived exosomes induced by mechanical strain promote human periodontal ligament stem cell proliferation and osteogenic differentiation via the miR-181b-5p/PTEN/AKT signaling pathway. Stem Cell Res Ther 11:295. https://doi.org/10.1186/s13287-020-01815-3
Guo F, Carter DE, Leask A (2011) Mechanical tension increases CCN2/CTGF expression and proliferation in gingival fibroblasts via a TGFβ-dependent mechanism. PLoS ONE 6:e19756. https://doi.org/10.1371/journal.pone.0019756
Wahlsten A, Rütsche D, Nanni M, Giampietro C, Biedermann T, Reichmann E et al (2021) Mechanical stimulation induces rapid fibroblast proliferation and accelerates the early maturation of human skin substitutes. Biomaterials 273:120779. https://doi.org/10.1016/j.biomaterials.2021.120779
Kang KS, Lee SJ, Lee HS, Moon W, Cho DW (2011) Effects of combined mechanical stimulation on the proliferation and differentiation of pre-osteoblasts. Exp Mol Med 43:367–373. https://doi.org/10.3858/emm.2011.43.6.040
Bleuel J, Zaucke F, Brüggemann GP, Niehoff A (2015) Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. PLoS ONE 10:e0119816. https://doi.org/10.1371/journal.pone.0119816
Castillo AB, Jacobs CR (2010) Mesenchymal stem cell mechanobiology. Curr Osteoporos Rep 8:98–104. https://doi.org/10.1007/s11914-010-0015-2
Luo W, Xiong W, Zhou J, Fang Z, Chen W, Fan Y et al (2011) Laminar shear stress delivers cell cycle arrest and anti-apoptosis to mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai) 43:210–216. https://doi.org/10.1093/abbs/gmr004
Li P, Ma YC, Sheng XY, Dong HT, Han H, Wang J et al (2012) Cyclic fluid shear stress promotes osteoblastic cells proliferation through ERK5 signaling pathway. Mol Cell Biochem 364:321–327. https://doi.org/10.1007/s11010-012-1233-y
Keller KC, Ding H, Tieu R, Sparks NR, Ehnes DD, Zur Nieden NI (2016) Wnt5a supports osteogenic lineage decisions in embryonic stem cells. Stem Cells Dev 25:1020–1032. https://doi.org/10.1089/scd.2015.0367
Agholme F, Isaksson H, Kuhstoss S, Aspenberg P (2011) The effects of Dickkopf-1 antibody on metaphyseal bone and implant fixation under different loading conditions. Bone 48:988–996. https://doi.org/10.1016/j.bone.2011.02.008
Ito R, Matsumiya T, Kon T, Narita N, Kubota K, Sakaki H et al (2014) Periosteum-derived cells respond to mechanical stretch and activate Wnt and BMP signaling pathways. Biomed Res 35:69–79. https://doi.org/10.2220/biomedres.35.69
Wang JH, Zhang Y, Li HY, Liu YY, Sun T (2016) Dickkopf-1 negatively regulates the expression of osteoprotegerin, a key osteoclastogenesis inhibitor, by sequestering Lrp6 in primary and metastatic lytic bone lesions. Medicine (Baltimore) 95:e3767. https://doi.org/10.1097/md.0000000000003767
Tortelote GG, Reis RR, de Almeida Mendes F, Abreu JG (2017) Complexity of the Wnt/β-catenin pathway: searching for an activation model. Cell Signal 40:30–43. https://doi.org/10.1016/j.cellsig.2017.08.008
Lee HL, Yi T, Baek K, Kwon A, Hwang HR, Qadir AS et al (2013) Tumor necrosis factor-α enhances the transcription of Smad ubiquitination regulatory factor 1 in an activating protein-1- and Runx2-dependent manner. J Cell Physiol 228:1076–1086. https://doi.org/10.1002/jcp.24256
Waiwut P, Shin MS, Yokoyama S, Saiki I, Sakurai H (2012) Gomisin A enhances tumor necrosis factor-α-induced G1 cell cycle arrest via signal transducer and activator of transcription 1-mediated phosphorylation of retinoblastoma protein. Biol Pharm Bull 35:1997–2003. https://doi.org/10.1248/bpb.b12-00450
Kook SH, Jang YS, Lee JC (2011) Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J Cell Biochem 112:2891–2901. https://doi.org/10.1002/jcb.23205
Mitsuhashi M, Yamaguchi M, Kojima T, Nakajima R, Kasai K (2011) Effects of HSP70 on the compression force-induced TNF-α and RANKL expression in human periodontal ligament cells. Inflamm Res 60:187–194. https://doi.org/10.1007/s00011-010-0253-x
Kim SJ, Park KH, Park YG, Lee SW, Kang YG (2013) Compressive stress induced the up-regulation of M-CSF, RANKL, TNF-α expression and the down-regulation of OPG expression in PDL cells via the integrin-FAK pathway. Arch Oral Biol 58:707–716. https://doi.org/10.1016/j.archoralbio.2012.11.003
Jaschke N, Hofbauer LC, Göbel A, Rachner TD (2020) Evolving functions of Dickkopf-1 in cancer and immunity. Cancer Lett 482:1–7. https://doi.org/10.1016/j.canlet.2020.03.031
Guo Y, Mishra A, Howland E, Zhao C, Shukla D, Weng T et al (2015) Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 126:2220–2229. https://doi.org/10.1182/blood-2015-02-622233
Chae WJ, Ehrlich AK, Chan PY, Teixeira AM, Henegariu O, Hao L et al (2016) The Wnt antagonist Dickkopf-1 promotes pathological type 2 cell-mediated inflammation. Immunity 44:246–258. https://doi.org/10.1016/j.immuni.2016.01.008
Lee JS, Ha L, Park JH, Lim JY (2012) Mechanical stretch suppresses BMP4 induction of stem cell adipogenesis via upregulating ERK but not through downregulating Smad or p38. Biochem Biophys Res Commun 418:278–283. https://doi.org/10.1016/j.bbrc.2012.01.010
Zhang T, Wen F, Wu Y, Goh GS, Ge Z, Tan LP et al (2015) Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 38:72–85. https://doi.org/10.1016/j.biomaterials.2014.10.010
Choi DH, Hwang HS (2019) Anti-inflammation activity of brazilin in TNF-α induced human psoriasis dermatitis skin model. Applied Biol Chem 62:1–9
Shaul YD, Seger R (2007) The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 1773:1213–1226
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (Nos. 81973684, 81500818, 81873334, and 82174358).
Funding
This work was supported by grants from the National Nature Science Foundation of China (Nos. 81973684, 82174358 and 81873334).
Author information
Authors and Affiliations
Contributions
YW: conceptualization, investigation, methodology, and writing of original draft. ZJ: investigation, methodology, and writing of original draft. DD: investigation, methodology, and writing of original draft. JY: investigation, methodology, and writing of original draft. ML: conceptualization, project administration, and writing – review & editing. LL: conceptualization, project administration, and writing – review & editing. YZ: methodology and visualization. WW: visualization, funding acquisition, project administration, and supervision. QH: funding acquisition, project administration, and supervision. YX: funding acquisition, project administration, and supervision.
Corresponding authors
Ethics declarations
Conflict of interest
No author has a financial relationship with a commercial firm that may pose a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Jing, Z., Deng, D. et al. Dkk-1–TNF-α crosstalk regulates MC3T3E1 pre-osteoblast proliferation and differentiation under mechanical stress through the ERK signaling pathway. Mol Cell Biochem 478, 2191–2206 (2023). https://doi.org/10.1007/s11010-022-04645-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04645-4