Abstract
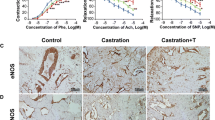
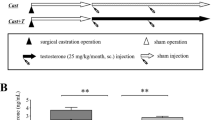
Erectile dysfunction (ED) is a major health problem affecting a large proportion of the general population. Testosterone also plays a key role in sexual dysfunction. In this study, we found that testosterone can inhibit cavernous fibrosis by affecting the expression of miR-22-3p, providing a new basis for research and treatment of ED. Old and young rats were used to study the effects of testosterone on cavernous fibrosis. Hematoxylin and eosin (HE) and Masson’s staining were used to observe the cavernous tissue. A luciferase assay was used to analyze the relationship between the miR-22-3p, TGFβR1, and Galectin-1 signaling pathways. CCK-8 and flow cytometry were used to detect the proliferation and apoptosis rates of cavernosum smooth muscle cells (CSMCs) following testosterone intervention. Immunohistochemical analysis was performed to examine the positive rate of caspase 3 and Ki67. IF was used to analyze the expression of collagen IV, MMP2, and α-SMA. The levels of GnRH, tT, LH, and F-TESTO in old rats increased after testosterone intervention. miR-22-3p inhibits the expression of TGFβR1 and Galectin-1. The protein expression of TGFβR1, Galectin-1, SMAD2, and p-SMAD2 was reduced by testosterone. The expression levels of α-SMA, collagen I, collagen IV, FN, and MMP2 in the cavernous tissues of old rats treated with testosterone were significantly reduced. The levels of caspase 3 and collagen IV decreased, and the levels of MMP2, Ki67, and α-SMA increased. Testosterone and miR-22-3p inhibit CSMC apoptosis and promote cell proliferation. Testosterone promoted the expression of miR-22-3p to interfere with the expression of the cavernous TGFβR1 and Galectin-1 signaling pathways. Testosterone can reduce cavernous fibrosis during the treatment of functional ED.






Similar content being viewed by others
Data availability
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
References
Montorsi F et al (2003) The ageing male and erectile dysfunction. BJU Int 92(5):516–520
Rogers RS et al (2003) Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int J Impot Res 15(1):26–37
Iacono F et al. (2012) Testosterone deficiency causes penile fibrosis and organic erectile dysfunction in aging men Evaluating association among Age TDS and ED. BMC Surg 12: 24
Zhang S, Zhao Y (2019) Lentinan protects cardiomyocytes against hypoxia-induced injury by regulation of microRNA-22/Sirt1. Artif Cells Nanomed Biotechnol 47(1):3938–3946
Tang Q et al (2018) Overexpression of miR-22 attenuates oxidative stress injury in diabetic cardiomyopathy via Sirt 1. Cardiovasc Ther 36(2):e12318
Huang W et al (2021) LncRNA Neat1 expedites the progression of liver fibrosis in mice through targeting miR-148a-3p and miR-22–3p to upregulate Cyth3. Cell Cycle 20:1–18
Zuo QF et al (2015) MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail. Cell Death Dis 6(11):e2000
Shao X et al (2017) Testosterone represses estrogen signaling by upregulating miR-22: a mechanism for imbalanced steroid hormone production in preeclampsia. Hypertension 69(4):721–730
You Y et al (2016) MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget 7(35):57099–57116
Hong Y et al (2016) MiR-22 may suppress fibrogenesis by targeting TGFβR I in cardiac fibroblasts. Cell Physiol Biochem 40(6):1345–1353
Hermenean A et al (2022) Galectin 1-a key player between tissue repair and fibrosis. Int J Mol Sci 23(10):5548
Wu D et al (2019) Galectin-1 promotes choroidal neovascularization and subretinal fibrosis mediated via epithelial-mesenchymal transition. Faseb j 33(2):2498–2513
Coppé JP et al (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Yousefzadeh MJ et al (2020) Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19(3):e13094
Gruenewald DA et al (2000) Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl 21(1):72–84
Gao L et al (2018) Effect of lysyl oxidase (LOX) on corpus cavernous fibrosis caused by ischaemic priapism. J Cell Mol Med 22(3):2018–2022
Zhang XH et al (2006) Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J Sex Med 3(2):253–64
Hwang I et al (2016) Testosterone modulates endothelial progenitor cells in rat corpus cavernosum. BJU Int 117(6):976–981
Cui K et al (2019) Testosterone preserves endothelial function through regulation of S1P1/Akt/FOXO3a signalling pathway in the rat corpus cavernosum. Andrologia 51(1):e13173
Mostafa T et al (2012) Effect of testosterone and frequent low-dose sildenafil/tadalafil on cavernous tissue oxidative stress of aged diabetic rats. Andrologia 44(6):411–415
Van den Broeck T et al (2020) Testosterone induces relaxation of human corpus cavernosum tissue of patients with erectile dysfunction. Sex Med 8(1):114–119
Chen FF et al (2019) Exogenous testosterone alleviates cardiac fibrosis and apoptosis via Gas6/Axl pathway in the senescent mice. Exp Gerontol 119:128–137
Miyauchi S et al (2017) Free testosterone concentration is inversely associated with markers of liver fibrosis in men with type 2 diabetes mellitus. Endocr J 64(12):1137–1142
Zhang G et al (2018) Amelioratory effects of testosterone propionate on age-related renal fibrosis via suppression of TGF-β1/smad signaling and activation of Nrf2-ARE signaling. Sci Rep 8(1):10726
Lopez DS et al (2017) Association of urinary phthalate metabolites with erectile dysfunction in racial and ethnic groups in the national health and nutrition examination survey 2001–2004. Am J Mens Health 11(3):576–584
Luo Y et al (2015) Sex hormones predict the incidence of erectile dysfunction: from a population-based prospective cohort study (FAMHES). J Sex Med 12(5):1165–1174
Ng E et al (2012) The influence of testosterone suppression and recovery on sexual function in men with prostate cancer: observations from a prospective study in men undergoing intermittent androgen suppression. J Urol 187(6):2162–2166
Paduch DA et al (2015) Testosterone replacement in androgen-deficient men with ejaculatory dysfunction: a randomized controlled trial. J Clin Endocrinol Metab 100(8):2956–2962
Cunningham GR et al (2016) Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab 101(8):3096–3104
Leask A (2007) TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res 74(2):207–212
Tan SM et al (2010) Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol 298(5):H1415–H1425
Lim MJ et al (2014) Induction of galectin-1 by TGF-β1 accelerates fibrosis through enhancing nuclear retention of Smad2. Exp Cell Res 326(1):125–135
Tang D et al (2018) Galectin-1 expression in activated pancreatic satellite cells promotes fibrosis in chronic pancreatitis/pancreatic cancer via the TGF-β1/Smad pathway. Oncol Rep 39(3):1347–1355
Zhang Z et al (2020) Aspirin inhibits endometrial fibrosis by suppressing the TGF-β1-Smad2/Smad3 pathway in intrauterine adhesions. Int J Mol Med 45(5):1351–1360
Xianyuan L et al (2019) Anti-renal fibrosis effect of asperulosidic acid via TGF-β1/smad2/smad3 and NF-κB signaling pathways in a rat model of unilateral ureteral obstruction. Phytomedicine 53:274–285
Feng F et al (2020) Tanshinone IIA attenuates silica-induced pulmonary fibrosis via inhibition of TGF-β1-Smad signaling pathway. Biomed Pharmacother 121:109586
Song KM et al (2014) Effectiveness of intracavernous delivery of adenovirus encoding Smad7 gene on erectile function in a mouse model of cavernous nerve injury. J Sex Med 11(1):51–63
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200(4):500–503
Eckes B et al (2000) Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol 19(4):325–332
Acknowledgements
This work was supported by National Natural Science Foundation of China (No.81973863), Traditional Chinese Medicine Scientific Research Program of Hunan Province (No.2022118), Scientific research project of Hunan Provincial Department of Education (No.22B1032) and Scientific Research Program of Hunan Provincial Health Commission (No.D202303107632).
Funding
This study was funded by National Natural Science Foundation of China (No.81973863), Traditional Chinese Medicine Scientific Research Program of Hunan Province (No.2022118), Scientific research project of Hunan Provincial Department of Education (No.22B1032) and Scientific Research Program of Hunan Provincial Health Commission (No.D202303107632).
Author information
Authors and Affiliations
Contributions
ZONGREN HU and QINGHU HE designed the whole study,as well as wrote the main manuscript text. YUANTING ZHANG analyzed the experimental data. JISONG CHEN and MIN LUO prepared figures 1-6. NENG WANG did an animal experiment. YINFU XIAO did cell experiment. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no commercial or other associations that might pose a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

11010_2022_4641_MOESM1_ESM.jpg
Testosterone may affect miR-22-3p by regulating AR. The expression of miR-22-3p decreased under the intervention of Bicalutamide. Supplementary file1 (JPG 213 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Z., Zhang, Y., Chen, J. et al. Testosterone attenuates senile cavernous fibrosis by regulating TGFβR1 and galectin-1 signaling pathways through miR-22-3p. Mol Cell Biochem 478, 1791–1802 (2023). https://doi.org/10.1007/s11010-022-04641-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04641-8