Abstract
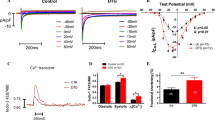
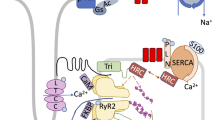
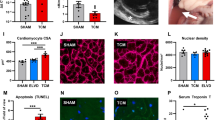
Premature ventricular contractions (PVCs) are the most frequent ventricular arrhythmias in the overall population. PVCs are known to acutely enhance contractility by the post-extrasystolic potentiation phenomenon, but over time persistent PVCs promote PVC-induced cardiomyopathy (PVC-CM), characterized by a reduction of the left ventricular (LV) ejection fraction. Ca2+ cycling in myocytes commands muscle contraction and in this process, SERCA2 leads the Ca2+ reuptake into the sarcoplasmic reticulum (SR) shaping cytosolic Ca2+ signal decay and muscle relaxation. Altered Ca2+ reuptake can contribute to the contractile dysfunction observed in PVC-CM. To better understand Ca2+ handling using our PVC-CM model (canines with 50% PVC burden for 12 weeks), SR-Ca2+ reuptake was investigated by measuring Ca2+ dynamics and analyzing protein expression. Kinetic analysis of Ca2+ reuptake in electrically paced myocytes showed a ~ 21 ms delay in PVC-CM compared to Sham in intact isolated myocytes, along with a ~ 13% reduction in SERCA2 activity assessed in permeabilized myocytes. Although these trends were not statistically significant between groups using hierarchical statistics, relaxation of myocytes following contraction was significantly slower in PVC-CM vs Sham myocytes. Western blot analyses indicate a 22% reduction in SERCA2 expression, a 23% increase in phospholamban (PLN) expression, and a 50% reduction in PLN phosphorylation in PVC-CM samples vs Sham. Computational analysis simulating a 20% decrease in SR-Ca2+ reuptake resulted in a ~ 22 ms delay in Ca2+ signal decay, consistent with the experimental result described above. In conclusion, SERCA2 and PLB alterations described above have a modest contribution to functional adaptations observed in PVC-CM.




Similar content being viewed by others
Data availability
Data are available on request to the authors.
References
Huizar JF, Ellenbogen KA, Tan AY, Kaszala K (2019) Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 73:2328–2344. https://doi.org/10.1016/j.jacc.2019.02.045
Huizar JF, Ellenbogen KA (2020) Is PVC-induced cardiomyopathy truly reversible?: A deep dive into questions that remain unanswered. JACC Clin Electrophysiol 6:1377–1380. https://doi.org/10.1016/j.jacep.2020.06.034
Huizar JF, Fisher SG, Ramsey FV, Kaszala K, Tan AY, Moore H, Koneru JN, Kron J, Padala SK, Ellenbogen KA, Singh SN (2021) Outcomes of premature ventricular contraction-cardiomyopathy in the veteran population: a secondary analysis of the CHF-STAT study. JACC Clin Electrophysiol 7:380–390. https://doi.org/10.1016/j.jacep.2020.08.028
Akkaya M, Roukoz H, Adabag S, Benditt DG, Anand I, Li JM, Zakharova M, Tholakanahalli V (2013) Improvement of left ventricular diastolic function and left atrial reverse remodeling after catheter ablation of premature ventricular complexes. J Interv Card Electrophysiol 38:179–185. https://doi.org/10.1007/s10840-013-9836-0
Yokokawa M, Good E, Crawford T, Chugh A, Pelosi F Jr, Latchamsetty R, Jongnarangsin K, Armstrong W, Ghanbari H, Oral H, Morady F, Bogun F (2013) Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm 10:172–175. https://doi.org/10.1016/j.hrthm.2012.10.011
Huizar JF, Tan AY, Kaszala K, Ellenbogen KA (2021) Clinical and translational insights on premature ventricular contractions and PVC-induced cardiomyopathy. Prog Cardiovasc Dis 66:17–27. https://doi.org/10.1016/j.pcad.2021.04.001
Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, Francis GS, Lenihan D, Lewis EF, McNamara DM, Pahl E, Vasan RS, Ramasubbu K, Rasmusson K, Towbin JA, Yancy C, American Heart Association Committee on Heart F, Transplantation of the Council on Clinical C, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on E, Prevention, Council on Quality of C and Outcomes R (2016) Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the american heart association. Circulation 134:e579–e646. https://doi.org/10.1161/CIR.0000000000000455
Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, Mezzaroma E, Chen Q, Kukreja RC, Hoke NN, Thacker LR 2nd, Ellenbogen KA, Wood MA (2011) Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol 4:543–549. https://doi.org/10.1161/CIRCEP.111.962381
Walters TE, Rahmutula D, Szilagyi J, Alhede C, Sievers R, Fang Q, Olgin J, Gerstenfeld EP (2018) Left ventricular dyssynchrony predicts the cardiomyopathy associated with premature ventricular contractions. J Am Coll Cardiol 72:2870–2882. https://doi.org/10.1016/j.jacc.2018.09.059
Yamada S, Lo LW, Chou YH, Lin WL, Chang SL, Lin YJ, Liu SH, Cheng WH, Tsai TY, Chen SA (2017) Beneficial effect of renal denervation on ventricular premature complex induced cardiomyopathy. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.004479
Torrado J, Kowlgi GN, Ramirez RJ, Balderas-Villalobos J, Jovin D, Parker C, Om E, Airapetov S, Kaszala K, Tan AY, Ellenbogen KA, Huizar JF (2021) Eccentric hypertrophy in an animal model of mid- and long-term premature ventricular contraction-induced cardiomyopathy. Heart Rhythm O2(2):80–88. https://doi.org/10.1016/j.hroo.2020.12.021
Wang Y, Eltit JM, Kaszala K, Tan A, Jiang M, Zhang M, Tseng GN, Huizar JF (2014) Cellular mechanism of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 11:2064–2072. https://doi.org/10.1016/j.hrthm.2014.07.022
Tada M, Yamada M, Kadoma M, Inui M, Ohmori F (1982) Calcium transport by cardiac sarcoplasmic reticulum and phosphorylation of phospholamban. Mol Cell Biochem 46:73–95. https://doi.org/10.1007/BF00236776
Bers DM (2000) Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res 87:275–281. https://doi.org/10.1161/01.res.87.4.275
Li L, Desantiago J, Chu G, Kranias EG, Bers DM (2000) Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol 278:H769–H779. https://doi.org/10.1152/ajpheart.2000.278.3.H769
Berlin JR, Bassani JW, Bers DM (1994) Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J 67:1775–1787. https://doi.org/10.1016/S0006-3495(94)80652-6
Bassani RA, Shannon TR, Bers DM (1998) Passive Ca2+ binding in ventricular myocardium of neonatal and adult rats. Cell Calcium 23:433–442. https://doi.org/10.1016/s0143-4160(98)90100-2
Penny WF, Hammond HK (2017) Randomized clinical trials of gene transfer for heart failure with reduced ejection fraction. Hum Gene Ther 28:378–384. https://doi.org/10.1089/hum.2016.166
Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S (2007) Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J 93:4083–4092. https://doi.org/10.1529/biophysj.107.114546
Ruchala I, Cabra V, Solis E Jr, Glennon RA, De Felice LJ, Eltit JM (2014) Electrical coupling between the human serotonin transporter and voltage-gated Ca(2+) channels. Cell Calcium 56:25–33. https://doi.org/10.1016/j.ceca.2014.04.003
Kubalova Z, Gyorke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Williams SC, Gyorke S (2004) Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J Physiol 561:515–524. https://doi.org/10.1113/jphysiol.2004.073940
Shannon TR, Guo T, Bers DM (2003) Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res 93:40–45. https://doi.org/10.1161/01.RES.0000079967.11815.19
Guo T, Ai X, Shannon TR, Pogwizd SM, Bers DM (2007) Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res 101:802–810. https://doi.org/10.1161/CIRCRESAHA.107.152140
Puglisi JL, Bers DM (2001) LabHEART: an interactive computer model of rabbit ventricular myocyte ion channels and Ca transport. Am J Physiol Cell Physiol 281:C2049–C2060. https://doi.org/10.1152/ajpcell.2001.281.6.C2049
Sikkel MB, Francis DP, Howard J, Gordon F, Rowlands C, Peters NS, Lyon AR, Harding SE, MacLeod KT (2017) Hierarchical statistical techniques are necessary to draw reliable conclusions from analysis of isolated cardiomyocyte studies. Cardiovasc Res 113:1743–1752. https://doi.org/10.1093/cvr/cvx151
Eisner DA (2021) Pseudoreplication in physiology: more means less. J Gen Physiol. https://doi.org/10.1085/jgp.202012826
Frank KF, Bolck B, Brixius K, Kranias EG, Schwinger RH (2002) Modulation of SERCA: implications for the failing human heart. Basic Res Cardiol 97(Suppl 1):I72–I78. https://doi.org/10.1007/s003950200033
Bovo E, Nikolaienko R, Bhayani S, Kahn D, Cao Q, Martin JL, Kuo IY, Robia SL, Zima AV (2019) Novel approach for quantification of endoplasmic reticulum Ca(2+) transport. Am J Physiol Heart Circ Physiol 316:H1323–H1331. https://doi.org/10.1152/ajpheart.00031.2019
Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ (2001) Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res 88:1059–1065. https://doi.org/10.1161/hh1001.091640
Layland J, Grieve DJ, Cave AC, Sparks E, Solaro RJ, Shah AM (2004) Essential role of troponin I in the positive inotropic response to isoprenaline in mouse hearts contracting auxotonically. J Physiol 556:835–847. https://doi.org/10.1113/jphysiol.2004.061176
Jiang M, Zhang M, Howren M, Wang Y, Tan A, Balijepalli RC, Huizar JF, Tseng GN (2016) JPH-2 interacts with Cai-handling proteins and ion channels in dyads: contribution to premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 13:743–752. https://doi.org/10.1016/j.hrthm.2015.10.037
Nediani C, Formigli L, Perna AM, Pacini A, Ponziani V, Modesti PA, Ibba-Manneschi L, Zecchi-Orlandini S, Fiorillo C, Cecchi C, Liguori P, Fratini G, Vanni S, Nassi P (2002) Biochemical changes and their relationship with morphological and functional findings in pig heart subjected to lasting volume overload: a possible role of acylphosphatase in the regulation of sarcoplasmic reticulum calcium pump. Basic Res Cardiol 97:469–478. https://doi.org/10.1007/s00395-002-0367-6
Simmerman HK, Jones LR (1998) Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev 78:921–947. https://doi.org/10.1152/physrev.1998.78.4.921
Nediani C, Celli A, Fiorillo C, Ponziani V, Giannini L, Nassi P (2003) Acylphosphatase interferes with SERCA2a-PLN association. Biochem Biophys Res Commun 301:948–951. https://doi.org/10.1016/s0006-291x(03)00078-0
Koss KL, Kranias EG (1996) Phospholamban: a prominent regulator of myocardial contractility. Circ Res 79:1059–1063. https://doi.org/10.1161/01.res.79.6.1059
Tan AY, Elharrif K, Cardona-Guarache R, Mankad P, Ayers O, Joslyn M, Das A, Kaszala K, Lin SF, Ellenbogen KA, Minisi AJ, Huizar JF (2020) Persistent proarrhythmic neural remodeling despite recovery from premature ventricular contraction-induced cardiomyopathy. J Am Coll Cardiol 75:1–13. https://doi.org/10.1016/j.jacc.2019.10.046
Salavatian S, Yamaguchi N, Hoang J, Lin N, Patel S, Ardell JL, Armour JA, Vaseghi M (2019) Premature ventricular contractions activate vagal afferents and alter autonomic tone: implications for premature ventricular contraction-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 317:H607–H616. https://doi.org/10.1152/ajpheart.00286.2019
Funding
This work was partially funded by National Institutes of Health grant R01 HL139874 (J.H.) and R01 AR068431 (M.S.), Department of Veterans Affairs grant 5I01BX004861 (J.H.), and a postdoctoral fellowship from the American Heart Association and the D.C. Women’s Board 836430 (J.B.V.).
Author information
Authors and Affiliations
Contributions
JE and JH: conceived and supervised the study. JE, JH, JB, and MS: designed the experiments. JB, JE, JH, JM, CL, RK, RR, AT, KK, and MS: performed experiments. JB, JE, RK, and JH: analyzed the data. JE: wrote the manuscript. JB, JH, and MS: made manuscript revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balderas-Villalobos, J., Medina-Contreras, J.M.L., Lynch, C. et al. Alterations of sarcoplasmic reticulum-mediated Ca2+ uptake in a model of premature ventricular contraction (PVC)-induced cardiomyopathy. Mol Cell Biochem 478, 1447–1456 (2023). https://doi.org/10.1007/s11010-022-04605-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04605-y