Abstract
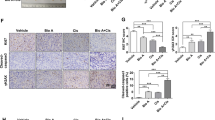
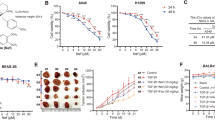
Protein arginine methyltransferase 5 (PRMT5) is overexpressed in lung carcinoma, which promotes tumor cell proliferation, survival, migration and invasion. Compound Kushen injection (CKI) is a mixture of natural compounds extracted from Kushen and Baituling, which are mainly used to stop in cancer pain and bleeding. Here we found that cell viability and colony formation were inhibited after the incubation of AMI-1. Meanwhile, AMI-1 suppressed cell migration, enhanced apoptosis, induced cell cycle arrest, inhibited PRMT5 expression and histone H3R8 and H4R3 symmetric di-methylation (H3R8me2s and H4R3me2s) accumulation, down-regulated the expression of eukaryotic translation initiation factor 4E (eIF4E) in lung carcinoma cells. Moreover, AMI-1 suppressed tumor growth, decreased H3R8me2s and H4R3me2s accumulation, down-regulated eIF4E expression and increased p53 expression in lung carcinoma xenografts of BALB/c nude mice. Of note, combined and CKI markedly enhanced the anticancer efficacy CKI in lung carcinoma. The above findings demonstrated that AMI-1 has established antineoplastic activity and this role may be associated with affecting the function of eIF4E via inhibiting PRMT5 activity or protein levels in lung carcinoma. This study highlights evidence of novel selective anticancer activity of AMI-1 in combination with CKI in lung carcinoma.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics (2020). CA Cancer J Clin 70(1):7–30. https://doi.org/10.3322/caac.21590
Kim D, Lee YS, Kim DH, Bae SC (2020) Lung cancer staging and associated genetic and epigenetic events. Mol Cells 43(1):1–9. https://doi.org/10.14348/molcells.2020.2246
Duruisseaux M, Esteller M (2018) Lung cancer epigenetics: from knowledge to applications. Semin Cancer Biol 51:116–128. https://doi.org/10.1016/j.semcancer.2017.09.005
Guccione E, Richard S (2019) The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol 20(10):642–657. https://doi.org/10.1038/s41580-019-0155-x
Jarrold J, Davies CC (2019) PRMTs and arginine methylation: cancer’s best-kept secret? Trends Mol Med 25(11):993–1009. https://doi.org/10.1016/j.molmed.2019.05.007
Kim H, Ronai ZA (2020) PRMT5 function and targeting in cancer. Cell Stress 4(8):199–215. https://doi.org/10.15698/cst2020.08.228
Stopa N, Krebs JE, Shechter D (2015) The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci 72(11):2041–2059. https://doi.org/10.1007/s00018-015-1847-9
Zakrzewicz D, Didiasova M, Krüger M, Giaimo BD, Borggrefe T, Mieth M, Hocke AC, Zakrzewicz A, Schaefer L, Preissner KT, Wygrecka M (2018) Protein arginine methyltransferase 5 mediates enolase-1 cell surface trafficking in human lung adenocarcinoma cells. Biochim Biophys Acta Mol Basis Dis 1864(5):1816–1827. https://doi.org/10.1016/j.bbadis.2018.02.021
Bao X, Zhao S, Liu T, Liu Y, Liu Y, Yang X (2013) Overexpression of PRMT5 promotes tumor cell growth and is associated with poor disease prognosis in epithelial ovarian cancer. J Histochem Cytochem 61(3):206–217. https://doi.org/10.1369/0022155413475452
Du W, Li D, Guo X, Li P, Li X, Tong S, Tong J, Kuang L, Liang D (2019) Circ-PRMT5 promotes gastric cancer progression by sponging miR-145 and miR-1304 to upregulate MYC. Artif Cells Nanomed Biotechnol 47(1):4120–4130. https://doi.org/10.1080/21691401.2019.1671857
Hartley AV, Wang B, Mundade R, Jiang G, Sun M, Wei H, Sun S, Liu Y, Lu T (2020) PRMT5-mediated methylation of YBX1 regulates NF-κB activity in colorectal cancer. Sci Rep 10(1):15934. https://doi.org/10.1038/s41598-020-72942-3
Li Y, Yang Y, Liu X, Long Y, Zheng Y (2019) PRMT5 promotes human lung cancer cell apoptosis via Akt/Gsk3β signaling induced by resveratrol. Cell Transplant 28(12):1664–1673. https://doi.org/10.1177/0963689719885083
Qin Y, Hu Q, Xu J, Ji S, Dai W, Liu W, Xu W, Sun Q, Zhang Z, Ni Q, Zhang B, Yu X, Xu X (2019) PRMT5 enhances tumorigenicity and glycolysis in pancreatic cancer via the FBW7/cMyc axis. Cell Commun Signal 17(1):30. https://doi.org/10.1186/s12964-019-0344-4
Radzisheuskaya A, Shliaha PV, Grinev V, Lorenzini E, Kovalchuk S, Shlyueva D, Gorshkov V, Hendrickson RC, Jensen ON, Helin K (2019) PRMT5 methylome profiling uncovers a direct link to splicing regulation in acute myeloid leukemia. Nat Struct Mol Biol 26(11):999–1012. https://doi.org/10.1038/s41594-019-0313-z
Shifteh D, Sapir T, Pahmer M, Haimowitz A, Goel S, Maitra R (2020) Protein arginine methyltransferase 5 as a therapeutic target for KRAS mutated colorectal cancer. Cancers (Basel) 12(8):2091. https://doi.org/10.3390/cancers12082091
Vinet M, Suresh S, Maire V, Monchecourt C, Némati F, Lesage L, Pierre F, Ye M, Lescure A, Brisson A, Meseure D, Nicolas A, Rigaill G, Marangoni E, Del Nery E, Roman-Roman S, Dubois T (2019) Protein arginine methyltransferase 5: a novel therapeutic target for triple-negative breast cancers. Cancer Med 8(5):2414–2428. https://doi.org/10.1002/cam4.2114
Zhang B, Dong S, Li Z, Lu L, Zhang S, Chen X, Cen X, Wu Y (2015) Targeting protein arginine methyltransferase 5 inhibits human hepatocellular carcinoma growth via the downregulation of beta-catenin. J Transl Med 13:349. https://doi.org/10.1186/s12967-015-0721-8
Lv JF, Hu L, Zhuo W, Zhang CM, Zhou HH, Fan L (2016) Epigenetic alternations and cancer chemotherapy response. Cancer Chemother Pharmacol 77(4):673–684. https://doi.org/10.1007/s00280-015-2951-0
Cheng D, Yadav N, King RW, Swanson MS, Weinstein EJ, Bedford MT (2004) Small molecule regulators of protein arginine methyltransferases. J Biol Chem 279(23):23892–23899. https://doi.org/10.1074/jbc.M401853200
Balcerczyk A, Rybaczek D, Wojtala M, Pirola L, Okabe J, El-Osta A (2016) Pharmacological inhibition of arginine and lysine methyltransferases induces nuclear abnormalities and suppresses angiogenesis in human endothelial cells. Biochem Pharmacol 121:18–32. https://doi.org/10.1016/j.bcp.2016.09.013
Zhang B, Dong S, Zhu R, Hu C, Hou J, Li Y, Zhao Q, Shao X, Bu Q, Li H, Wu Y, Cen X, Zhao Y (2015) Targeting protein arginine methyltransferase 5 inhibits colorectal cancer growth by decreasing arginine methylation of eIF4E and FGFR3. Oncotarget 6(26):22799–22811. https://doi.org/10.18632/oncotarget.4332
Janisiak J, Kopytko P, Tkacz M, Rogińska D, Perużyńska M, Machaliński B, Pawlik A, Tarnowski M (2021) Protein Arginine Methyltransferase (PRMT) inhibitors-AMI-1 and SAH are effective in attenuating rhabdomyosarcoma growth and proliferation in cell cultures. Int J Mol Sci 22(15):8023. https://doi.org/10.3390/ijms22158023
Li TW, Zhang Q, Oh P, Xia M, Chen H, Bemanian S, Lastra N, Circ M, Moyer MP, Mato JM, Aw TY, Lu SC (2009) S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol Pharmacol 76(1):192–200. https://doi.org/10.1124/mol.108.054411
Andreu-Pérez P, Hernandez-Losa J, Moliné T, Gil R, Grueso J, Pujol A, Cortés J, Avila MA, Recio JA (2010) Methylthioadenosine (MTA) inhibits melanoma cell proliferation and in vivo tumor growth. BMC Cancer 10:265. https://doi.org/10.1186/1471-2407-10-265
Mehta A, Dobersch S, Romero-Olmedo AJ, Barreto G (2015) Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev 34(2):229–241. https://doi.org/10.1007/s10555-015-9563-3
Yang Y, Bedford MT (2013) Protein arginine methyltransferases and cancer. Nat Rev Cancer 13(1):37–50. https://doi.org/10.1038/nrc3409
Kim H, Kim H, Feng Y, Li Y, Tamiya H, Tocci S, Ronai ZA (2020) PRMT5 control of cGAS/STING and NLRC5 pathways defines melanoma response to antitumor immunity. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaz5683
Litzler LC, Zahn A, Meli AP, Hébert S, Patenaude AM, Methot SP, Sprumont A, Bois T, Kitamura D, Costantino S, King IL, Kleinman CL, Richard S, Di Noia JM (2019) PRMT5 is essential for B cell development and germinal center dynamics. Nat Commun 10(1):22. https://doi.org/10.1038/s41467-018-07884-6
Wang Y, Hu W, Yuan Y (2018) Protein arginine methyltransferase 5 (PRMT5) as an anticancer target and its inhibitor discovery. J Med Chem 61(21):9429–9441. https://doi.org/10.1021/acs.jmedchem.8b00598
Bandyopadhyay S, Harris DP, Adams GN, Lause GE, McHugh A, Tillmaand EG, Money A, Willard B, Fox PL, Dicorleto PE (2012) HOXA9 methylation by PRMT5 is essential for endothelial cell expression of leukocyte adhesion molecules. Mol Cell Biol 32(7):1202–1213. https://doi.org/10.1128/mcb.05977-11
Hou Z, Peng H, Ayyanathan K, Yan KP, Langer EM, Longmore GD, Rauscher FJ 3rd (2008) The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol Cell Biol 28(10):3198–3207. https://doi.org/10.1128/MCB.01435-07
Lim JH, Lee YM, Lee G, Choi YJ, Lim BO, Kim YJ, Choi DK, Park JW (2014) PRMT5 is essential for the eIF4E-mediated 5’-cap dependent translation. Biochem Biophys Res Commun 452(4):1016–1021. https://doi.org/10.1016/j.bbrc.2014.09.033
Siddiqui N, Sonenberg N (2015) Signalling to eIF4E in cancer. Biochem Soc Trans 43(5):763–772. https://doi.org/10.1042/bst20150126
Zhang HH, Li R, Li YJ, Yu XX, Sun QN, Li AY, Kong Y (2020) eIF4E-related miR-320a and miR-340-5p inhibit endometrial carcinoma cell metastatic capability by preventing TGF-β1-induced epithelial-mesenchymal transition. Oncol Rep 43(2):447–460. https://doi.org/10.3892/or.2019.7437
Carroll M, Borden KL (2013) The oncogene eIF4E: using biochemical insights to target cancer. J Interferon Cytokine Res 33(5):227–238. https://doi.org/10.1089/jir.2012.0142
Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B, La Thangue NB (2008) Arginine methylation regulates the p53 response. Nat Cell Biol 10(12):1431–1439. https://doi.org/10.1038/ncb1802
Fedoriw A, Rajapurkar SR, O’Brien S, Gerhart SV, Mitchell LH, Adams ND, Rioux N, Lingaraj T, Ribich SA, Pappalardi MB, Shah N, Laraio J, Liu Y, Butticello M, Carpenter CL, Creasy C, Korenchuk S, McCabe MT, McHugh CF, Nagarajan R, Wagner C, Zappacosta F, Annan R, Concha NO, Thomas RA, Hart TK, Smith JJ, Copeland RA, Moyer MP, Campbell J, Stickland K, Mills J, Jacques-O’Hagan S, Allain C, Johnston D, Raimondi A, Porter Scott M, Waters N, Swinger K, Boriack-Sjodin A, Riera T, Shapiro G, Chesworth R, Prinjha RK, Kruger RG, Barbash O, Mohammad HP (2019) Anti-tumor activity of the type I PRMT inhibitor, GSK3368715, synergizes with PRMT5 inhibition through MTAP Loss. Cancer Cell 36(1):100-114.e125. https://doi.org/10.1016/j.ccell.2019.05.014
Srour N, Mersaoui SY, Richard S (2019) M-TAP dance: targeting PRMT1 and PRMT5 family members to push cancer cells over the edge. Cancer Cell 36(1):3–5. https://doi.org/10.1016/j.ccell.2019.06.004
Takai H, Masuda K, Sato T, Sakaguchi Y, Suzuki T, Suzuki T, Koyama-Nasu R, Nasu-Nishimura Y, Katou Y, Ogawa H, Morishita Y, Kozuka-Hata H, Oyama M, Todo T, Ino Y, Mukasa A, Saito N, Toyoshima C, Shirahige K, Akiyama T (2014) 5-Hydroxymethylcytosine plays a critical role in glioblastomagenesis by recruiting the CHTOP-methylosome complex. Cell Rep 9(1):48–60. https://doi.org/10.1016/j.celrep.2014.08.071
Wang W, You RL, Qin WJ, Hai LN, Fang MJ, Huang GH, Kang RX, Li MH, Qiao YF, Li JW, Li AP (2015) Anti-tumor activities of active ingredients in compound Kushen injection. Acta Pharmacol Sin 36(6):676–679. https://doi.org/10.1038/aps.2015.24
Nourmohammadi S, Aung TN, Cui J, Pei JV, De Ieso ML, Harata-Lee Y, Qu Z, Adelson DL, Yool AJ (2019) Effect of compound Kushen injection, a natural compound mixture, and its identified chemical components on migration and invasion of colon, brain, and breast cancer cell lines. Front Oncol 9:314. https://doi.org/10.3389/fonc.2019.00314
Wang KX, Du GH, Qin XM, Gao L (2021) Compound Kushen injection intervenes metabolic reprogramming and epithelial-mesenchymal transition of HCC via regulating β-catenin/c-Myc signaling. Phytomedicine 93:153781. https://doi.org/10.1016/j.phymed.2021.153781
Wu H, Wang L, Zhan X, Wang B, Wu J, Zhou A (2020) A UPLC-Q-TOF/MS-based plasma metabolomics approach reveals the mechanism of compound Kushen injection-based intervention against non-small cell lung cancer in Lewis tumor-bearing mice. Phytomedicine 76:153259. https://doi.org/10.1016/j.phymed.2020.153259
Jin Y, Yang Q, Liang L, Ding L, Liang Y, Zhang D, Wu B, Yang T, Liu H, Huang T, Shen H, Tu H, Pan Y, Wei Y, Yang Y, Zhou F (2018) Compound kushen injection suppresses human acute myeloid leukaemia by regulating the Prdxs/ROS/Trx1 signalling pathway. J Exp Clin Cancer Res 37(1):277. https://doi.org/10.1186/s13046-018-0948-3
Xu W, Lin H, Zhang Y, Chen X, Hua B, Hou W, Qi X, Pei Y, Zhu X, Zhao Z, Yang L (2011) Compound Kushen injection suppresses human breast cancer stem-like cells by down-regulating the canonical Wnt/β-catenin pathway. J Exp Clin Cancer Res 30(1):103. https://doi.org/10.1186/1756-9966-30-103
Zhao Z, Liao H, Ju Y (2016) Effect of compound Kushen injection on T-cell subgroups and natural killer cells in patients with locally advanced non-small-cell lung cancer treated with concomitant radiochemotherapy. J Tradit Chin Med 36(1):14–18. https://doi.org/10.1016/s0254-6272(16)30002-4
Zhang J, Qu Z, Yao H, Sun L, Harata-Lee Y, Cui J, Aung TN, Liu X, You R, Wang W, Hai L, Adelson DL, Lin L (2019) An effective drug sensitizing agent increases gefitinib treatment by down regulating PI3K/Akt/mTOR pathway and up regulating autophagy in non-small cell lung cancer. Biomed Pharmacother 118:109169. https://doi.org/10.1016/j.biopha.2019.109169
Bajbouj K, Ramakrishnan RK, Saber-Ayad M, Omar HA, Saheb Sharif-Askari N, Shafarin J, Elmoselhi AB, Ihmaid A, AlHaj Ali S, Alalool A, Abdullah R, Hamid Q (2021) PRMT5 selective inhibitor enhances therapeutic efficacy of cisplatin in lung cancer cells. Int J Mol Sci 22(11):6131. https://doi.org/10.3390/ijms22116131
Acknowledgements
Not applicable.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
ZBL designed the experiments, supervised the project and analyzed the data; YRY, DSH and ZJH performed the experiments, analyzed the data and wrote the manuscript; ZSH and MGL performed the experiments; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All animal studies were conducted in accordance with the protocol and guidelines approved by the Animal Ethics Committee of School of Basic Medical Sciences, Lanzhou University (Approval No. jcyxy20190505).
Informed consent
All the authors meet the qualifications for authorship and had an opportunity to read and comment the manuscript. All authors support publication of the manuscript in Molecular and Cellular Biochemistry.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, R., Dong, S., Zhang, J. et al. Downregulation of PRMT5 by AMI-1 enhances therapeutic efficacy of compound kushen injection in lung carcinoma in vitro and in vivo. Mol Cell Biochem 478, 1031–1044 (2023). https://doi.org/10.1007/s11010-022-04577-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04577-z