Abstract
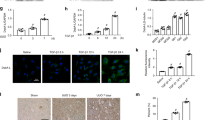
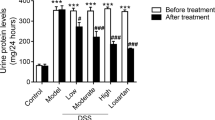
The focal segmental glomerulosclerosis (FSGS) is one of the most frequent glomerulopathy in the world, being considered a significative public health problem worldwide. The disease is characterized by glomerular loss mainly due to inflammation process and collagen fibers deposition. STAT-3 is a transcription factor associated with cell differentiation, migration and proliferation and in renal cells it has been related with fibrosis, acting on the progression of the lesion. Considering this perspective, the present study evaluated the involvement of STAT-3 molecule in an experimental model of FSGS induced by Doxorubicin (DOX). DOX mimics primary FSGS by causing both glomerular and tubular lesions and the inhibition of the STAT3 pathway leads to a decrease in fibrosis and attenuation of kidney damage. We described here a novel FSGS experimental model in a strain of genetically heterogeneous mice which resembles the reality of FSGS patients. DOX-injected mice presented elevated indices of albuminuria and glycosuria, that were significantly reduced in animals treated with a STAT-3 inhibitor (STATTIC), in addition with a decrease of some inflammatory molecules. Moreover, we detected that SOCS-3 (a regulator of STAT family) was up-regulated only in STATTIC-treated mice. Finally, histopathological analyzes showed that DOX-treated group had a significant increase in a tubulointerstitial fibrosis and tubular necrosis, which were not identified in both control and STATTIC groups. Thus, our results indicate that STAT-3 pathway possess an important role in experimental FSGS induced by DOX and may be an important molecule to be further investigated.





Similar content being viewed by others
Data availability
The data analyzed during the study is not publically available but can be available on request to corresponding author.
References
Bahiense-Oliveira M, Saldanha LB, Mota EL, Penna DO, Barros RT, Romao-Junior JE (2004) Primary glomerular diseases in Brazil (1979–1999): is the frequency of focal and segmental glomerulosclerosis increasing? Clin Nephrol 61:90–97. https://doi.org/10.5414/cnp61090
D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC (2013) Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8:399–406. https://doi.org/10.2215/CJN.06100612
Polito MG, de Moura LA, Kirsztajn GM (2010) An overview on frequency of renal biopsy diagnosis in Brazil: clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant 25:490–496. https://doi.org/10.1093/ndt/gfp355
Shabaka A, Tato Ribera A, Fernandez-Juarez G (2020) Focal segmental glomerulosclerosis: state-of-the-art and clinical perspective. Nephron 144:413–427. https://doi.org/10.1159/000508099
Hommos MS, De Vriese AS, Alexander MP, Sethi S, Vaughan L, Zand L, Bharucha K, Lepori N, Rule AD, Fervenza FC (2017) The incidence of primary vs secondary focal segmental glomerulosclerosis: a clinicopathologic study. Mayo Clin Proc 92:1772–1781. https://doi.org/10.1016/j.mayocp.2017.09.011
Angioi A, Pani A (2016) FSGS: from pathogenesis to the histological lesion. J Nephrol 29:517–523. https://doi.org/10.1007/s40620-016-0333-2
Sprangers B, Meijers B, Appel G (2016) FSGS: diagnosis and diagnostic work-up. Biomed Res Int 2016:4632768. https://doi.org/10.1155/2016/4632768
Uffing A, Perez-Saez MJ, Mazzali M, Manfro RC, Bauer AC, de Sottomaior DF, O’Shaughnessy MM, Cheng XS, Chin KK, Ventura CG, Agena F, David-Neto E, Mansur JB, Kirsztajn GM, Tedesco-Silva H Jr, Neto GMV, Arias-Cabrales C, Buxeda A, Bugnazet M, Jouve T, Malvezzi P, Akalin E, Alani O, Agrawal N, La Manna G, Comai G, Bini C, Muhsin SA, Riella MC, Hokazono SR, Farouk SS, Haverly M, Mothi SS, Berger SP, Cravedi P, Riella LV (2020) Recurrence of FSGS after kidney transplantation in adults. Clin J Am Soc Nephrol 15:247–256. https://doi.org/10.2215/CJN.08970719
Lafayette RA (2020) Facing the vexing problem of recurrent FSGS after kidney transplantation. Clin J Am Soc Nephrol 15:171–173. https://doi.org/10.2215/CJN.14841219
Ponticelli C, Glassock RJ (2010) Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol 5:2363–2372. https://doi.org/10.2215/CJN.06720810
Lee VW, Harris DC (2011) Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16:30–38. https://doi.org/10.1111/j.1440-1797.2010.01383.x
Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M (1986) Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int 29:502–510. https://doi.org/10.1038/ki.1986.28
Xie L, Koukos G, Barck K, Foreman O, Lee WP, Brendza R, Eastham-Anderson J, McKenzie BS, Peterson A, Carano RAD (2019) Micro-CT imaging and structural analysis of glomeruli in a model of Adriamycin-induced nephropathy. Am J Physiol Renal Physiol 316:F76–F89. https://doi.org/10.1152/ajprenal.00331.2018
Shakeel S, Mubarak M, Kazi JI, Jafry N, Ahmed E (2013) Frequency and clinicopathological characteristics of variants of primary focal segmental glomerulosclerosis in adults presenting with nephrotic syndrome. J Nephropathol 2:28–35. https://doi.org/10.5812/nephropathol.8959
Shakeel S, Mubarak M, Kazi JI (2014) Frequency and clinicopathological correlations of histopathological variants of idiopathic focal segmental glomerulosclerosis in nephrotic adolescents. J Pak Med Assoc 64:322–326
Fogo AB (2003) Animal models of FSGS: lessons for pathogenesis and treatment. Semin Nephrol 23:161–171. https://doi.org/10.1053/snep.2003.50015
Abbate M, Zoja C, Remuzzi G (2006) How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17:2974–2984. https://doi.org/10.1681/ASN.2006040377
Li Y, Zhou H, Li Y, Han L, Song M, Chen F, Shang G, Wang D, Wang Z, Zhang W, Zhong M (2019) PTPN2 improved renal injury and fibrosis by suppressing STAT-induced inflammation in early diabetic nephropathy. J Cell Mol Med 23:4179–4195. https://doi.org/10.1111/jcmm.14304
Liu Q, Liang X, Liang M, Qin R, Qin F, Wang X (2020) Ellagic acid ameliorates renal ischemic-reperfusion injury through NOX4/JAK/STAT signaling pathway. Inflammation 43:298–309. https://doi.org/10.1007/s10753-019-01120-z
Liu Y, Feng Q, Miao J, Wu Q, Zhou S, Shen W, Feng Y, Hou FF, Liu Y, Zhou L (2020) C-X-C motif chemokine receptor 4 aggravates renal fibrosis through activating JAK/STAT/GSK3beta/beta-catenin pathway. J Cell Mol Med 24:3837–3855. https://doi.org/10.1111/jcmm.14973
Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, Hartman J, Wang W, Kretzler M, Lafayette RA (2020) JAK-STAT activity in peripheral blood cells and kidney tissue in IgA nephropathy. Clin J Am Soc Nephrol 15:973–982. https://doi.org/10.2215/CJN.11010919
Huang JS, Lee YH, Chuang LY, Guh JY, Hwang JY (2015) Cinnamaldehyde and nitric oxide attenuate advanced glycation end products-induced the Jak/STAT signaling in human renal tubular cells. J Cell Biochem 116:1028–1038. https://doi.org/10.1002/jcb.25058
O’Leary R, Penrose H, Miyata K, Satou R (2016) Macrophage-derived IL-6 contributes to ANG II-mediated angiotensinogen stimulation in renal proximal tubular cells. Am J Physiol Renal Physiol 310:F1000–F1007. https://doi.org/10.1152/ajprenal.00482.2015
Zhang B, Guo Z, Lai S, Chen H (2020) Interference with miR-210 alleviated renal injury in septic rats by inhibiting JAK-STAT pathway. Inflammation. https://doi.org/10.1007/s10753-020-01283-0
Gu Y, Mohammad IS, Liu Z (2020) Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol Lett 19:2585–2594. https://doi.org/10.3892/ol.2020.11394
Abualsunun WA, Sahin C, Cummins CL, Piquette-Miller M (2020) Essential role of STAT-3 dependent NF-kappaB activation on IL-6-mediated downregulation of hepatic transporters. Eur J Pharm Sci 143:105151. https://doi.org/10.1016/j.ejps.2019.105151
Yang YL, Liu P, Li D, Yang Q, Li B, Jiang XJ (2020) Stat-3 signaling promotes cell proliferation and metastasis of gastric cancer through PDCD4 downregulation. Kaohsiung J Med Sci 36:244–249. https://doi.org/10.1002/kjm2.12159
Chuang PY, He JC (2010) JAK/STAT signaling in renal diseases. Kidney Int 78:231–234. https://doi.org/10.1038/ki.2010.158
Bienaime F, Muorah M, Yammine L, Burtin M, Nguyen C, Baron W, Garbay S, Viau A, Broueilh M, Blanc T, Peters D, Poli V, Anglicheau D, Friedlander G, Pontoglio M, Gallazzini M, Terzi F (2016) Stat3 controls tubulointerstitial communication during CKD. J Am Soc Nephrol 27:3690–3705. https://doi.org/10.1681/ASN.2015091014
Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S (2010) A novel STAT3 inhibitor, S3I–201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78:257–268. https://doi.org/10.1038/ki.2010.154
Jeansson M, Bjorck K, Tenstad O, Haraldsson B (2009) Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol 20:114–122. https://doi.org/10.1681/ASN.2007111205
Cybulsky AV, Takano T, Papillon J, Guillemette J, Herzenberg AM, Kennedy CR (2010) Podocyte injury and albuminuria in mice with podocyte-specific overexpression of the Ste20-like kinase, SLK. Am J Pathol 177:2290–2299. https://doi.org/10.2353/ajpath.2010.100263
de Almeida DC, Bassi EJ, Azevedo H, Anderson L, Origassa CS, Cenedeze MA, de Andrade-Oliveira V, Felizardo RJ, da Silva RC, Hiyane MI, Semedo P, Dos Reis MA, Moreira-Filho CA, Verjovski-Almeida S, Pacheco-Silva A, Camara NO (2016) A regulatory miRNA-mRNA network is associated with tissue repair induced by mesenchymal stromal cells in acute kidney injury. Front Immunol 7:645. https://doi.org/10.3389/fimmu.2016.00645
Ahmad SF, Ansari MA, Nadeem A, Zoheir KMA, Bakheet SA, Alsaad AMS, Al-Shabanah OA, Attia SM (2017) STA-21, a STAT-3 inhibitor, attenuates the development and progression of inflammation in collagen antibody-induced arthritis. Immunobiology 222:206–217. https://doi.org/10.1016/j.imbio.2016.10.001
Pedroza M, Le TT, Lewis K, Karmouty-Quintana H, To S, George AT, Blackburn MR, Tweardy DJ, Agarwal SK (2016) STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation. FASEB J 30:129–140. https://doi.org/10.1096/fj.15-273953
Campbell KN, He JC (2014) Can biomarkers of disease activity guide treatment in FSGS? Clin J Am Soc Nephrol 9:1507–1509. https://doi.org/10.2215/CJN.07170714
Cravedi P, Kopp JB, Remuzzi G (2013) Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant 13:266–274. https://doi.org/10.1111/ajt.12045
Korbet SM (2012) Treatment of primary FSGS in adults. J Am Soc Nephrol 23:1769–1776. https://doi.org/10.1681/ASN.2012040389
Sethna CB, Gipson DS (2014) Treatment of FSGS in children. Adv Chronic Kidney Dis 21:194–199. https://doi.org/10.1053/j.ackd.2014.01.010
Zheng Z, Schmidt-Ott KM, Chua S, Foster KA, Frankel RZ, Pavlidis P, Barasch J, D’Agati VD, Gharavi AG (2005) A Mendelian locus on chromosome 16 determines susceptibility to doxorubicin nephropathy in the mouse. Proc Natl Acad Sci USA 102:2502–2507. https://doi.org/10.1073/pnas.0409786102
Pereira RL, Reis VO, Semedo P, Buscariollo BN, Donizetti-Oliveira C, Cenedeze MA, Soares MF, Pacheco-Silva A, Savage PB, Camara NO, Keller AC (2012) Invariant natural killer T cell agonist modulates experimental focal and segmental glomerulosclerosis. PLoS ONE 7:e32454. https://doi.org/10.1371/journal.pone.0032454
Scott RP, Quaggin SE (2015) Review series: the cell biology of renal filtration. J Cell Biol 209:199–210. https://doi.org/10.1083/jcb.201410017
Garbers C, Aparicio-Siegmund S, Rose-John S (2015) The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol 34:75–82. https://doi.org/10.1016/j.coi.2015.02.008
Calabrese LH, Rose-John S (2014) IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol 10:720–727. https://doi.org/10.1038/nrrheum.2014.127
Hunter CA, Jones SA (2015) IL-6 as a keystone cytokine in health and disease. Nat Immunol 16:448–457. https://doi.org/10.1038/ni.3153
Isobe S, Ohashi N, Katahashi N, Ishigaki S, Tsuji N, Tsuji T, Kato A, Fujigaki Y, Shimizu A, Yasuda H (2017) Focal segmental glomerulosclerosis associated with cutaneous and systemic plasmacytosis. CEN Case Rep 6:206–209. https://doi.org/10.1007/s13730-017-0276-z
Yoshioka K, Takemura T, Murakami K, Okada M, Yagi K, Miyazato H, Matsushima K, Maki S (1993) In situ expression of cytokines in IgA nephritis. Kidney Int 44:825–833. https://doi.org/10.1038/ki.1993.317
Carow B, Rottenberg ME (2014) SOCS3, a major regulator of infection and inflammation. Front Immunol 5:58. https://doi.org/10.3389/fimmu.2014.00058
Meng XM (2019) Inflammatory mediators and renal fibrosis. Adv Exp Med Biol 1165:381–406. https://doi.org/10.1007/978-981-13-8871-2_18
Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, Hartman J, Wang W, Kretzler M, Lafayette RA (2018) JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int 94:795–808. https://doi.org/10.1016/j.kint.2018.05.022
Funding
Thabata Caroline de Oliveira Santos was supported by a grant of CAPES (Coordenação de aperfeiçoamento de pessoal de nível superior) - Brazil.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TCOS, GP. The first draft of the manuscript was written by TCOS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All experimental procedures were carried out following the ethical principles established by the Experimental Brazilian Council (COBEA) and approved by the local Animal Ethics Committee (protocol n°957).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Santos, T.C., Pereira, G., Coutinho, A.G.G. et al. STAT-3 signaling role in an experimental model of nephropathy induced by doxorubicin. Mol Cell Biochem 478, 981–989 (2023). https://doi.org/10.1007/s11010-022-04574-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04574-2