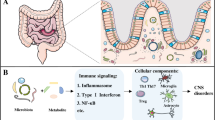
Abstract
Previous studies have found that intracorporal short-chain fatty acids (SCFAs), as the main metabolites of the gut microbiota, play important roles in the intestinal physiology and immune function. Along with the in-depth study of the brain-gut axis, the attention to the roles of SCFAs in central nervous system (CNS) has been raised. It has been found that SCFAs function in CNS diseases by regulating inflammatory response, neuronal apoptosis, oxidative stress, the integrity of the blood–brain barrier (BBB) and so on. Here, the changes, the effects and the mechanisms of different SCFA as individual or mixture in different CNS diseases were summarized. It is expected to lead to increased interest in SCFAs studies as an important regulator in CNS diseases and provide feasible suggestions based on SCFAs for the therapy of CNS diseases in the future.

Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Cummings JH, Pomare EW, Branch WJ et al (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28(10):1221–1227. https://doi.org/10.1136/gut.28.10.1221
Freedman SN, Shahi SK, Mangalam AK (2018) The “gut feeling”: breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics 15(1):109–125. https://doi.org/10.1007/s13311-017-0588-x
Niccolai E, Baldi S, Ricci F et al (2019) Evaluation and comparison of short chain fatty acids composition in gut diseases. World J Gastroenterol 25(36):5543–5558. https://doi.org/10.3748/wjg.v25.i36.5543
Duncan SH, Belenguer A, Holtrop G et al (2007) Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73(4):1073–1078. https://doi.org/10.1128/aem.02340-06
De Filippis F, Pellegrini N, Vannini L et al (2016) High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65(11):1812–1821. https://doi.org/10.1136/gutjnl-2015-309957
Park J, Wang Q, Wu Q et al (2019) Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci Rep 9(1):8837. https://doi.org/10.1038/s41598-019-45311-y
Hoyles L, Snelling T, Umlai UK et al (2018) Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome 6(1):55. https://doi.org/10.1186/s40168-018-0439-y
Liu J, Wang F, Liu S et al (2017) Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci 381:176–181. https://doi.org/10.1016/j.jns.2017.08.3235
Sadler R, Cramer JV, Heindl S et al (2020) Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci 40(5):1162–1173. https://doi.org/10.1523/jneurosci.1359-19.2019
Zheng L, Kelly CJ (2017) Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J Immunol 199(8):2976–2984. https://doi.org/10.4049/jimmunol.1700105
Tong LC, Wang Y, Wang ZB et al (2016) Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharmacol 7:253. https://doi.org/10.3389/fphar.2016.00253
Kominsky DJ, Campbell EL, Ehrentraut SF et al (2014) IFN-γ-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J Immunol 192(3):1267–1276. https://doi.org/10.4049/jimmunol.1301757
Kumari R, Ahuja V, Paul J (2013) Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J Gastroenterol 19(22):3404–3414. https://doi.org/10.3748/wjg.v19.i22.3404
Wang W, Chen L, Zhou R et al (2014) Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol 52(2):398–406. https://doi.org/10.1128/jcm.01500-13
Takahashi K, Nishida A, Fujimoto T et al (2016) Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 93(1):59–65. https://doi.org/10.1159/000441768
Arpaia N, Campbell C, Fan X et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480):451–455. https://doi.org/10.1038/nature12726
Furusawa Y, Obata Y, Fukuda S et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446–450. https://doi.org/10.1038/nature12721
Chang PV, Hao L, Offermanns S et al (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 111(6):2247–2252. https://doi.org/10.1073/pnas.1322269111
Wu W, Sun M, Chen F et al (2017) Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 10(4):946–956. https://doi.org/10.1038/mi.2016.114
Soret R, Chevalier J, De Coppet P et al (2010) Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138(5):1772–1782. https://doi.org/10.1053/j.gastro.2010.01.053
Hamer HM, Jonkers DM, Bast A et al (2009) Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 28(1):88–93. https://doi.org/10.1016/j.clnu.2008.11.002
Tang Y, Chen Y, Jiang H et al (2011) Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ 18(4):602–618. https://doi.org/10.1038/cdd.2010.117
Qiao CM, Sun MF, Jia XB et al (2020) Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res 387(1):111772. https://doi.org/10.1016/j.yexcr.2019.111772
Thangaraju M, Cresci GA, Liu K et al (2009) GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69(7):2826–2832. https://doi.org/10.1158/0008-5472.can-08-4466
Donohoe DR, Holley D, Collins LB et al (2014) A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov 4(12):1387–1397. https://doi.org/10.1158/2159-8290.cd-14-0501
Mangalam A, Shahi SK, Luckey D et al (2017) Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep 20(6):1269–1277. https://doi.org/10.1016/j.celrep.2017.07.031
Brissette CA, Houdek HM, Floden AM et al (2012) Acetate supplementation reduces microglia activation and brain interleukin-1β levels in a rat model of Lyme neuroborreliosis. J Neuroinflammation 9:249. https://doi.org/10.1186/1742-2094-9-249
Erny D, Hrabě de Angelis AL, Jaitin D et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–977. https://doi.org/10.1038/nn.4030
Huuskonen J, Suuronen T, Nuutinen T et al (2004) Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol 141(5):874–880. https://doi.org/10.1038/sj.bjp.0705682
Jaworska J, Ziemka-Nalecz M, Sypecka J et al (2017) The potential neuroprotective role of a histone deacetylase inhibitor, sodium butyrate, after neonatal hypoxia-ischemia. J Neuroinflammation 14(1):34. https://doi.org/10.1186/s12974-017-0807-8
Patnala R, Arumugam TV, Gupta N et al (2017) HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol 54(8):6391–6411. https://doi.org/10.1007/s12035-016-0149-z
Soliman ML, Combs CK, Rosenberger TA (2013) Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J Neuroimmune Pharmacol 8(1):287–300. https://doi.org/10.1007/s11481-012-9426-4
Sun J, Wang F, Li H et al (2015) Neuroprotective effect of sodium butyrate against cerebral ischemia/reperfusion injury in mice. Biomed Res Int. https://doi.org/10.1155/2015/395895
Kidd SK, Schneider JS (2010) Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res 1354:172–178. https://doi.org/10.1016/j.brainres.2010.07.041
Ying M, Xu R, Wu X et al (2006) Sodium butyrate ameliorates histone hypoacetylation and neurodegenerative phenotypes in a mouse model for DRPLA. J Biol Chem 281(18):12580–12586. https://doi.org/10.1074/jbc.M511677200
Chou AH, Chen SY, Yeh TH et al (2011) HDAC inhibitor sodium butyrate reverses transcriptional downregulation and ameliorates ataxic symptoms in a transgenic mouse model of SCA3. Neurobiol Dis 41(2):481–488. https://doi.org/10.1016/j.nbd.2010.10.019
Steffan JS, Bodai L, Pallos J et al (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413(6857):739–743. https://doi.org/10.1038/35099568
Harrison IF, Smith AD, Dexter DT (2018) Pathological histone acetylation in Parkinson’s disease: Neuroprotection and inhibition of microglial activation through SIRT 2 inhibition. Neurosci Lett 666:48–57. https://doi.org/10.1016/j.neulet.2017.12.037
Alquézar C, Barrio E, Esteras N et al (2015) Targeting cyclin D3/CDK6 activity for treatment of Parkinson’s disease. J Neurochem 133(6):886–897. https://doi.org/10.1111/jnc.13070
Barichello T, Generoso JS, Simões LR et al (2015) Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol Neurobiol 52(1):734–740. https://doi.org/10.1007/s12035-014-8914-3
Li Y, Perry T, Kindy MS et al (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A 106(4):1285–1290. https://doi.org/10.1073/pnas.0806720106
Perez-Pardo P, Dodiya HB, Engen PA et al (2019) Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68(5):829–843. https://doi.org/10.1136/gutjnl-2018-316844
Unger MM, Spiegel J, Dillmann KU et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72. https://doi.org/10.1016/j.parkreldis.2016.08.019
Zheng J, Zheng SJ, Cai WJ et al (2019) Stable isotope labeling combined with liquid chromatography-tandem mass spectrometry for comprehensive analysis of short-chain fatty acids. Anal Chim Acta 1070:51–59. https://doi.org/10.1016/j.aca.2019.04.021
Zhang L, Wang Y, Xiayu X et al (2017) Altered gut microbiota in a mouse model of Alzheimer’s disease. J Alzheimers Dis 60(4):1241–1257. https://doi.org/10.3233/jad-170020
Zeng Q, Junli G, Liu X et al (2019) Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem Int. https://doi.org/10.1016/j.neuint.2019.104468
Chitrala KN, Guan H, Singh NP et al (2017) CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. Eur J Immunol 47(7):1188–1199. https://doi.org/10.1002/eji.201646792
Gong J, Qiu W, Zeng Q et al (2019) Lack of short-chain fatty acids and overgrowth of opportunistic pathogens define dysbiosis of neuromyelitis optica spectrum disorders: a Chinese pilot study. Mult Scler 25(9):1316–1325. https://doi.org/10.1177/1352458518790396
Chen R, Xu Y, Wu P et al (2019) Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res 148:104403. https://doi.org/10.1016/j.phrs.2019.104403
Sampson TR, Debelius JW, Thron T et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(6):1469-1480.e12. https://doi.org/10.1016/j.cell.2016.11.018
Park J, Kim M, Kang SG et al (2015) Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8(1):80–93. https://doi.org/10.1038/mi.2014.44
Kong Y, Jiang B, Luo X (2018) Gut microbiota influences Alzheimer’s disease pathogenesis by regulating acetate in Drosophila model. Future Microbiol 13:1117–1128. https://doi.org/10.2217/fmb-2018-0185
Smith MD, Bhatt DP, Geiger JD et al (2014) Acetate supplementation modulates brain adenosine metabolizing enzymes and adenosine A2A receptor levels in rats subjected to neuroinflammation. J Neuroinflammation 11:99. https://doi.org/10.1186/1742-2094-11-99
Liu J, Li H, Gong T et al (2020) Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer’s Disease via upregulating GPR41 and Inhibiting ERK/JNK/NF-κB. J Agric Food Chem 68(27):7152–7161. https://doi.org/10.1021/acs.jafc.0c02807
Haghikia A, Jörg S, Duscha A et al (2015) Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43(4):817–829. https://doi.org/10.1016/j.immuni.2015.09.007
Duscha A, Gisevius B, Hirschberg S et al (2020) Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 180(6):1067-1080.e16. https://doi.org/10.1016/j.cell.2020.02.035
Cheng Y, Mai Q, Zeng X et al (2019) Propionate relieves pentylenetetrazol-induced seizures, consequent mitochondrial disruption, neuron necrosis and neurological deficits in mice. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2019.08.009
Rane P, Shields J, Heffernan M et al (2012) The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacology 62(7):2409–2412. https://doi.org/10.1016/j.neuropharm.2012.01.026
Keshavarzian A, Green SJ, Engen PA et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30(10):1351–1360. https://doi.org/10.1002/mds.26307
St Laurent R, O’Brien LM, Ahmad ST (2013) Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience 246:382–390. https://doi.org/10.1016/j.neuroscience.2013.04.037
Srivastav S, Neupane S, Bhurtel S et al (2019) Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem 69:73–86. https://doi.org/10.1016/j.jnutbio.2019.03.021
Sharma S, Taliyan R, Singh S (2015) Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: modulation of histone deacetylase activity. Behav Brain Res 291:306–314. https://doi.org/10.1016/j.bbr.2015.05.052
Paiva I, Pinho R, Pavlou MA et al (2017) Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum Mol Genet 26(12):2231–2246. https://doi.org/10.1093/hmg/ddx114
Jin H, Kanthasamy A, Harischandra DS et al (2014) Histone hyperacetylation up-regulates protein kinase Cδ in dopaminergic neurons to induce cell death: relevance to epigenetic mechanisms of neurodegeneration in Parkinson disease. J Biol Chem 289(50):34743–34767. https://doi.org/10.1074/jbc.M114.576702
Qiao CM, Sun MF, Jia XB et al (2020) Sodium butyrate exacerbates Parkinson’s disease by aggravating neuroinflammation and colonic inflammation in MPTP-induced mice model. Neurochem Res 45(9):2128–2142. https://doi.org/10.1007/s11064-020-03074-3
Wu X, Chen PS, Dallas S et al (2008) Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol 11(8):1123–1134. https://doi.org/10.1017/s1461145708009024
Jarrett JT, Berger EP, Lansbury PT Jr (1993) The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry 32(18):4693–4697. https://doi.org/10.1021/bi00069a001
Mroczko B, Groblewska M, Litman-Zawadzka A (2019) The role of protein misfolding and tau oligomers (TauOs) in Alzheimer’s disease (AD). Int J Mol Sci. https://doi.org/10.3390/ijms20194661
Govindarajan N, Agis-Balboa RC, Walter J et al (2011) Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis 26(1):187–197. https://doi.org/10.3233/jad-2011-110080
Fernando W, Martins IJ, Morici M et al (2020) Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer’s disease transgenic mouse model at an early disease stage. J Alzheimers Dis 74(1):91–99. https://doi.org/10.3233/jad-190120
Marizzoni M, Cattaneo A, Mirabelli P et al (2020) Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in alzheimer’s disease. J Alzheimers Dis 78(2):683–697. https://doi.org/10.3233/jad-200306
Sun J, Xu J, Yang B et al (2020) Effect of clostridium butyricum against microglia-mediated neuroinflammation in Alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201900636
Ferrante RJ, Kubilus JK, Lee J et al (2003) Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci 23(28):9418–9427. https://doi.org/10.1523/jneurosci.23-28-09418.2003
Minamiyama M, Katsuno M, Adachi H et al (2004) Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 13(11):1183–1192. https://doi.org/10.1093/hmg/ddh131
Chou AH, Chen YL, Hu SH et al (2014) Polyglutamine-expanded ataxin-3 impairs long-term depression in Purkinje neurons of SCA3 transgenic mouse by inhibiting HAT and impairing histone acetylation. Brain Res 1583:220–229. https://doi.org/10.1016/j.brainres.2014.08.019
Naia L, Cunha-Oliveira T, Hayden MR et al (2017) Histone deacetylase inhibitors protect against pyruvate dehydrogenase dysfunction in Huntington’s disease. J Neurosci 37(10):2776–2794. https://doi.org/10.1523/jneurosci.2006-14.2016
Ziemka-Nalecz M, Jaworska J, Sypecka J et al (2017) Sodium butyrate, a histone deacetylase inhibitor, exhibits neuroprotective/neurogenic effects in a rat model of neonatal hypoxia-ischemia. Mol Neurobiol 54(7):5300–5318. https://doi.org/10.1007/s12035-016-0049-2
Kim HJ, Rowe M, Ren M et al (2007) Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 321(3):892–901. https://doi.org/10.1124/jpet.107.120188
Li D, Bai X, Jiang Y et al (2020) Butyrate alleviates PTZ-induced mitochondrial dysfunction, oxidative stress and neuron apoptosis in mice via Keap1/Nrf2/HO-1 pathway. Brain Res Bull 168:25–35. https://doi.org/10.1016/j.brainresbull.2020.12.009
Chen T, Noto D, Hoshino Y et al (2019) Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation 16(1):165. https://doi.org/10.1186/s12974-019-1552-y
Li H, Sun J, Wang F et al (2016) Sodium butyrate exerts neuroprotective effects by restoring the blood–brain barrier in traumatic brain injury mice. Brain Res 1642:70–78. https://doi.org/10.1016/j.brainres.2016.03.031
Brustovetsky T, Purl K, Young A et al (2004) Dearth of glutamate transporters contributes to striatal excitotoxicity. Exp Neurol 189(2):222–230. https://doi.org/10.1016/j.expneurol.2004.03.021
Wu DM, Wang S, Wen X et al (2019) Suppression of microRNA-342-3p increases glutamate transporters and prevents dopaminergic neuron loss through activating the Wnt signaling pathway via p21-activated kinase 1 in mice with Parkinson’s disease. J Cell Physiol 234(6):9033–9044. https://doi.org/10.1002/jcp.27577
Johnson J Jr, Pajarillo EAB, Taka E et al (2018) Valproate and sodium butyrate attenuate manganese-decreased locomotor activity and astrocytic glutamate transporters expression in mice. Neurotoxicology 64:230–239. https://doi.org/10.1016/j.neuro.2017.06.007
Ho RH, Chan JCY, Fan H et al (2017) In silico and in vitro interactions between short chain fatty acids and human histone deacetylases. Biochemistry 56(36):4871–4878. https://doi.org/10.1021/acs.biochem.7b00508
Luu M, Pautz S, Kohl V et al (2019) The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun 10(1):760. https://doi.org/10.1038/s41467-019-08711-2
Ho L, Ono K, Tsuji M et al (2018) Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 18(1):83–90. https://doi.org/10.1080/14737175.2018.1400909
Pryde SE, Duncan SH, Hold GL et al (2002) The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 217(2):133–139. https://doi.org/10.1111/j.1574-6968.2002.tb11467.x
Stewart ML, Slavin JL (2006) Molecular weight of guar gum affects short-chain fatty acid profile in model intestinal fermentation. Mol Nutr Food Res 50(10):971–976. https://doi.org/10.1002/mnfr.200600024
Funding
This study was supported by National Natural Science Foundation of China (82171429).
Author information
Authors and Affiliations
Contributions
YD contributed to writing and revision of the manuscript. Professor CC provided valuable comments for manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, Y., Cui, C. The role of short-chain fatty acids in central nervous system diseases. Mol Cell Biochem 477, 2595–2607 (2022). https://doi.org/10.1007/s11010-022-04471-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04471-8