Abstract
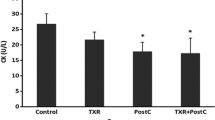
Coronary endothelial dysfunction is a major cause of ischemia–reperfusion (I/R) injury. Trehalose, a natural disaccharide, has been reported to ameliorate endothelial dysfunction during aging by activating endothelial nitric oxide synthase (eNOS); however, its role in I/R injury is unknown. This study evaluated the effects of trehalose preconditioning on cardiac and coronary endothelial function after I/R. Langendorff-perfused rat hearts underwent 30 min of global ischemia followed by 80 min of reperfusion with or without trehalose preconditioning. Rate pressure product (RPP) and coronary flow (CF) were measured during reperfusion. Perivascular edema was assessed by hematoxylin and eosin staining. Myocardial oxidative stress and apoptosis were evaluated by immunohistochemistry and TUNEL staining, respectively. eNOS dimerization was determined by western blotting. An eNOS inhibitor was used to examine the role of eNOS. Trehalose preconditioning showed a higher recovery rate after I/R as indicated by high RPP (control vs. trehalose, 28 ± 6% vs. 46 ± 9%; P = 0.017, Cohen’s d = 2.3) and CF values (35 ± 10% vs. 55 ± 9%; P = 0.025, d = 1.7). Furthermore, trehalose preconditioning reduced perivascular edema, myocardial oxidative stress, and apoptosis. The eNOS dimerization ratio was increased by trehalose (1.2 ± 0.2 vs. 1.6 ± 0.2; P = 0.023, d = 2.1), which was associated with the recovery of RPP and CF. These effects of trehalose were abolished by the eNOS inhibitor. Trehalose preconditioning showed protective effects on cardiac and coronary endothelial function after I/R through the eNOS signaling pathway.








Similar content being viewed by others
Data availability
The datasets generated during the current study will be shared on reasonable request to the corresponding author.
References
Dobson GP, Faggian G, Onorati F, Vinten-Johansen J (2013) Hyperkalemic cardioplegia for adult and pediatric surgery: end of an era? Front Physiol 4:228. https://doi.org/10.3389/fphys.2013.00228
Beyersdorf F (2009) The use of controlled reperfusion strategies in cardiac surgery to minimize ischaemia/reperfusion damage. Cardiovasc Res 83:262–268. https://doi.org/10.1093/cvr/cvp110
Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GGL, Possati G, Gaudino M (2004) Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardio-thorac Surg 25:304–311. https://doi.org/10.1016/j.ejcts.2003.12.003
Wu MY, Yiang GT, Liao WT, Tsai APY, Cheng YL, Cheng PW, Li CY, Li CJ (2018) Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 46:1650–1667. https://doi.org/10.1159/000489241
Heusch G (2015) Molecular basis of cardioprotection signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116:674–699. https://doi.org/10.1161/CIRCRESAHA.116.305348
Minamino T (2012) Cardioprotection from ischemia/reperfusion injury: basic and translational research. Circ J 76:1074–1082. https://doi.org/10.1253/circj.CJ-12-0132
Gunata M, Parlakpinar H (2021) A review of myocardial ischaemia/reperfusion injury: pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem Funct 39:190–217. https://doi.org/10.1002/cbf.3587
Heusch G, Rassaf T (2016) Time to give up on cardioprotection?: a critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ Res 119:676–695. https://doi.org/10.1161/CIRCRESAHA.116.308736
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. The Lancet 381:166–175. https://doi.org/10.1016/S0140-6736(12)60916-7
Heusch G (2016) The coronary circulation as a target of cardioprotection. Circ Res 118:1643–1658. https://doi.org/10.1161/CIRCRESAHA.116.308640
Hausenloy DJ, Chilian W, Crea F, Davidson SM, Ferdinandy P, Garcia-Dorado D, van Royen N, Schulz R, Hausch G (2019) The coronary circulation in acute myocardial ischaemia/reperfusion injury: a target for cardioprotection. Cardiovasc Res 115:1143–1155. https://doi.org/10.1093/cvr/cvy286
Singhal AK, Symons JD, Boudina S, Jaishy B, Shiu YT (2010) Role of endothelial cells in myocardial ischemia-reperfusion injury. Vasc Dis Prev 7:1–14
Hosseinpour-Moghaddam K, Caraglia M, Sahebkar A (2018) Autophagy induction by trehalose: molecular mechanisms and therapeutic impacts. J Cell Physiol 233:6524–6543. https://doi.org/10.1002/jcp.26583
Kumar SK, Prakash T, Vetriselvan M, Mani KP (2021) Trehalose protects the endothelium from cadmium-induced dysfunction. Cell Biol Int 45:957–964. https://doi.org/10.1002/cbin.11539
Larocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR (2012) Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590:3305–3316. https://doi.org/10.1113/jphysiol.2012.229690
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837. https://doi.org/10.1093/eurheartj/ehr304
Merry Lindsey XL, Bolli R, Canty JM, Du X-J, Frangogiannis NG, Frantz S et al (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiolo Heart Circ Physiol 314:812–838. https://doi.org/10.1152/ajpheart.00335.2017
Bell RM, Mocanu MM, Yellon DM (2011) Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 50:940–950. https://doi.org/10.1016/j.yjmcc.2011.02.018
Méndez-Carmona N, Wyss RK, Arnold M, Joachimbauer A, Segiser A, Fiedler GM, Carrel TP, Tevarearai Stahel HT, Longnus SL (2019) Differential effects of ischemia/reperfusion on endothelial function and contractility in donation after circulatory death. J Heart Lung Transpl 38:767–777. https://doi.org/10.1016/j.healun.2019.03.004
Que W, Hu X, Fujino M, Terayama H, Sakabe K, Fukunishi N, Zhu P, Yi SQ, Yamada Y, Zhong L, Li XK (2020) Prolonged cold ischemia time in mouse heart transplantation using supercooling preservation. Transplantation. https://doi.org/10.1097/TP.0000000000003089
Petersen DR, Doorn JA (2004) Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med 37:937–945. https://doi.org/10.1016/j.freeradbiomed.2004.06.012
Saraste A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 45:528–537
Klionsky DJ, Kamal Abdel-Aziz A, Abdelfatah S, Abdellatif M, Abdoli A, Abel S et al (2021) Guidelines for the use and interpretation of assays for monitoring autophagy(4th edition). Autophagy 17:1–382
Yang Y-M, Huang A, Kaley G, Sun D (2009) eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297:1829–1836. https://doi.org/10.1152/ajpheart.00230.2009.-Endothelial
Sullivan GM, Feinn R (2012) Using effect size—or why the P value is not enough. J Grad Med Educ 4:279–282. https://doi.org/10.4300/jgme-d-12-00156.1
Gödecke A, Heinicke T, Kamkin A, Kiseleva I, Strasser RH, Decking UKM, Stumpe T, Isenberg G, Schrader J (2001) Inotropic response to b-adrenergic receptor stimulation and anti-adrenergic effect of ACh in endothelial NO synthase-deficient mouse hearts. J Physiol 532:195–204
Daiber A, Xia N, Steven S, Oelze M, Hanf A, Kröller-Schön S, Mönzel T, Li H (2019) New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int J Mol Sci 20:187. https://doi.org/10.3390/ijms20010187
Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ (2014) Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal 20:3040–3077. https://doi.org/10.1089/ars.2013.5566
Jlzd C (2007) Myocardial ischemia results in tetrahydrobiopterin (BH 4) oxidation with impaired endothelial function ameliorated by BH 4. PNAS 104:15081–15086
Wang C, Qiao S, Hong L, Sun J, Che T, An J, Camara AKS (2020) NOS cofactor tetrahydrobiopterin contributes to anesthetic preconditioning induced myocardial protection in the isolated ex vivo rat heart. Int J Mol Med 45:615–622. https://doi.org/10.3892/ijmm.2019.4445
Farah C, Nascimento A, Bolea G, Meyer G, Gayrard S, Lacampagne A, Gazorla O, Reboul C (2017) Key role of endothelium in the eNOS-dependent cardioprotection with exercise training. J Mol Cell Cardiol 102:26–30. https://doi.org/10.1016/j.yjmcc.2016.11.008
Zhang JX, Qu XL, Chu P, Xie DJ, Zhu LL, Chao YL, Li L, Zhang JJ, Chen SL (2018) Low shear stress induces vascular eNOS uncoupling via autophagy-mediated eNOS phosphorylation. Biochim Biophys Acta Mol Cell Res 1865:709–720. https://doi.org/10.1016/j.bbamcr.2018.02.005
Liu Z, Chen D, Chen X, Bian F, Qin W, Gao N, Xiao Y, Li J, Pflugfelder SC, Li DQ (2020) Trehalose induces autophagy against inflammation by activating TFEB signaling pathway in human corneal epithelial cells exposed to hyperosmotic stress. Investig Ophthalmol Vis Sci 61:26. https://doi.org/10.1167/IOVS.61.10.26
Palmieri M, Pal R, Nelvagal HR, Lotfi P, Stinnett GR, Seymour ML, Chaudhury A, Bajaj L, Bondar VV, Bremner L, Saleem U, Tse DY, Sanagasetti D, Wu SM, Neilson JR, Pereira FA, Pautler RG, Rodney GG, Cooper JD, Sardiello M (2017) MTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun 8:14338. https://doi.org/10.1038/ncomms14338
Wu H, Chen H, Zheng Z, Li J, Ding J, Huang Z, Jia C, Shen Z, Bao G, Wu L, Al Mamun A, Xu Hm Gao W, Zhou K (2019) Trehalose promotes the survival of random-pattern skin flaps by TFEB mediated autophagy enhancement. Cell Death Dis 10:483. https://doi.org/10.1038/s41419-019-1704-0
Mizunoe Y, Kobayashi M, Sudo Y, Watanabe S, Yasukawa H, Natori D, Hoshino A, Negishi A, Okita N, Komatsu M, Higami Y (2018) Trehalose protects against oxidative stress by regulating the Keap1–Nrf2 and autophagy pathways. Redox Biol 15:115–124. https://doi.org/10.1016/j.redox.2017.09.007
Liu S, Yang Y, Gao H, Zhou N, Wang P, Zhang Y, Zhang A, Jia Z, Huang S (2020) Trehalose attenuates renal ischemia-reperfusion injury by enhancing autophagy and inhibiting oxidative stress and inflammation. Am J Physiol Renal Physiol 318:994–1005. https://doi.org/10.1152/ajprenal.00568.2019.-Re
Jiang J, Xu H (2020) Trehalose alleviates oxygen-glucose deprivation induced myocardial injury through the autophagy-lysosome pathway. Int J Clin Exp Med 13:6606–6615
Leucker TM, Ge ZD, Procknow J, Liu Y, Shi Y, Bienengraeber M, Warltier DC, Kersten JR (2013) Impairment of endothelial-myocardial interaction increases the susceptibility of cardiomyocytes to ischemia/reperfusion injury. PLoS ONE. https://doi.org/10.1371/journal.pone.0070088
Brutsaert DL (2003) Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83:59–115. https://doi.org/10.1152/physrev.00017.2002.-Experimental
Di Napoli P, Di Crecchio A, Contegiacomo G, Tiloca P, Taccardi A, Maggi A et al (1998) Endothelial protective effect of verapamil against acute myocardial contractile dysfunction in isolated working rat hearts subjected to global ischemia. Ann N Y Acad Sci 853:311–315
Ohtake S, Wang YJ (2011) Trehalose: current use and future applications. J Pharm Sci 100:2020–2053. https://doi.org/10.1002/jps.22458
Chen FS, Nakamura T, Wada H (2004) Development of new organ preservation solutions in Kyoto University. Yonsei Med J 45:1107–1114
Acknowledgements
The authors thank Editage for English language editing. We also thank Sapporo General Pathology Laboratory for technical assistance with the experiments.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by KS and YS. Manuscript revision was performed by SW. The first draft of the manuscript was written by KS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All applicable international and national guidelines for the care and use of animals were followed. And all experimental procedures were conducted according to the Hokkaido university manual for implementing animal experimentation. No human studies were carried out by the authors for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suno, K., Shingu, Y. & Wakasa, S. Protective effects of trehalose preconditioning on cardiac and coronary endothelial function through eNOS signaling pathway in a rat model of ischemia–reperfusion injury. Mol Cell Biochem 477, 2403–2414 (2022). https://doi.org/10.1007/s11010-022-04451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04451-y