Abstract
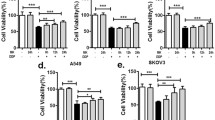
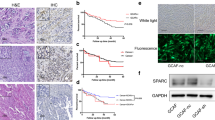
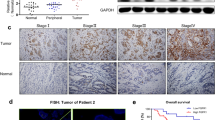
This study aimed to investigate the role of cancer-associated fibroblast (CAF)-derived midkine (MK) in cisplatin (DDP) resistance. The primary cultures of CAFs and non-cancer fibroblasts (NFs) were isolated and purified. The DDP-resistant gastric cancer (GC) cells were cultured with CAF-conditioned medium. QRT-PCR and Elisa assays were employed to determine MK expression. The expression of ST7-AS1 was measured by qRT-PCR. The impact of CAFs, MK, and ST7-AS1 silencing on DDP resistance was determined by MTT and Annexin V/PI staining assay. Expression of EMT markers and PI3K/AKT was determined by Western blot and qRT-PCR. The role of MK in DDP resistance was confirmed in a xenograft model. Incubation with CAF-conditioned medium increased the IC50 to DDP. Also, incubation with CAF-conditioned medium increased cell viability, reduced cell apoptosis, and promoted EMT in DDP-resistant GC cells, which were all blocked with MK neutralization antibody treatment. MK increased the DDP resistance and upregulated the expression of ST7-AS1 in DDP-resistant GC cells. Additionally, ST7-AS1 knockdown increased the sensitivity to DDP by inhibiting EMT. Moreover, ST7-AS1 knockdown significantly decreased the phosphorylation of PI3K and AKT, and suppressed EMT, which were restored by MK addition. Finally, MK promoted tumor growth and DDP resistance in a mice model bearing the SGC-7901/DDP xenografts. CAF-derived MK promotes EMT-mediated DDP resistance via upregulation of ST7-AS1 and activation of PI3K/AKT pathway.
Graphical abstract






Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP (2018) Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 10:239–248. https://doi.org/10.2147/CMAR.S149619
Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X (2016) Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Dev Ther 10:1885–1895. https://doi.org/10.2147/DDDT.S106412
Koberle B, Tomicic MT, Usanova S, Kaina B (2010) Cisplatin resistance: preclinical findings and clinical implications. Biochim Biophys Acta 1806:172–182. https://doi.org/10.1016/j.bbcan.2010.07.004
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14:1014–1022. https://doi.org/10.1038/ni.2703
Li J, Guan J, Long X, Wang Y, Xiang X (2016) mir-1-mediated paracrine effect of cancer-associated fibroblasts on lung cancer cell proliferation and chemoresistance. Oncol Rep 35:3523–3531
Navab R, Strumpf D, Bandarchi B, Zhu CQ, Pintilie M, Ramnarine VR, Ibrahimov E, Radulovich N, Leung L, Barczyk M, Panchal D, To C, Yun JJ, Der S, Shepherd FA, Jurisica I, Tsao MS (2011) Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci USA 108:7160–7165. https://doi.org/10.1073/pnas.1014506108
Zhang D, Ding L, Li Y, Ren J, Shi G, Wang Y, Zhao S, Ni Y, Hou Y (2017) Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci Rep 7:16231. https://doi.org/10.1038/s41598-017-13431-y
Lorente M, Torres S, Salazar M, Carracedo A, Hernandez-Tiedra S, Rodriguez-Fornes F, Garcia-Taboada E, Melendez B, Mollejo M, Campos-Martin Y, Lakatosh SA, Barcia J, Guzman M, Velasco G (2011) Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death Differ 18:959–973. https://doi.org/10.1038/cdd.2010.170
Mirkin BL, Clark S, Zheng X, Chu F, White BD, Greene M, Rebbaa A (2005) Identification of midkine as a mediator for intercellular transfer of drug resistance. Oncogene 24:4965–4974. https://doi.org/10.1038/sj.onc.1208671
Suda K, Murakami I, Yu H, Kim J, Tan AC, Mizuuchi H, Rozeboom LM, Ellison K, Rivard CJ, Mitsudomi T, Hirsch FR (2018) CD44 facilitates epithelial to mesenchymal transition phenotypic change at acquisition of resistance to EGFR kinase inhibitors in lung cancer. Mol Cancer Ther. https://doi.org/10.1158/1535-7163.MCT-17-1279
He H, Ni J, Huang J (2014) Molecular mechanisms of chemoresistance in osteosarcoma. Oncol Lett 7:1352–1362
Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W (2009) Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 69:5820–5828. https://doi.org/10.1158/0008-5472.CAN-08-2819
Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S, Squire J, Park PC, Feilotter H (2012) EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer 12:91. https://doi.org/10.1186/1471-2407-12-91
Zhao G, Nie Y, Lv M, He L, Wang T, Hou Y (2012) ERβ-mediated estradiol enhances epithelial mesenchymal transition of lung adenocarcinoma through increasing transcription of midkine. Mol Endocrinol 26:1304–1315. https://doi.org/10.1210/me.2012-1028
Wang LL, Zhang XH, Zhang X, Chu JK (2016) MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT). Eur Rev Med Pharmacol Sci 20:1733–1739
Huang Y, Hoque MO, Wu F, Trink B, Sidransky D, Ratovitski EA (2008) Midkine induces epithelial–mesenchymal transition through Notch2/Jak2-Stat3 signaling in human keratinocytes. Cell Cycle 7:1613–1622. https://doi.org/10.4161/cc.7.11.5952
Gungor C, Zander H, Effenberger KE, Vashist YK, Kalinina T, Izbicki JR, Yekebas E, Bockhorn M (2011) Notch signaling activated by replication stress-induced expression of midkine drives epithelial–mesenchymal transition and chemoresistance in pancreatic cancer. Cancer Res 71:5009–5019. https://doi.org/10.1158/0008-5472.CAN-11-0036
Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W (2019) Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res 9:1354–1366
Zhou Y, Sun W, Qin Z, Guo S, Kang Y, Zeng S, Yu L (2021) LncRNA regulation: new frontiers in epigenetic solutions to drug chemoresistance. Biochem Pharmacol 189:114228. https://doi.org/10.1016/j.bcp.2020.114228
Hu R-H, Zhang Z-T, Wei H-X, Ning L, Ai J-S, Li W-H, Zhang H, Wang S-Q (2020) LncRNA ST7-AS1, by regulating miR-181b-5p/KPNA4 axis, promotes the malignancy of lung adenocarcinoma. Cancer Cell Int 20:568. https://doi.org/10.1186/s12935-020-01652-7
Qin H, Xu J, Gong L, Jiang B, Zhao W (2019) The long noncoding RNA ST7-AS1 promotes laryngeal squamous cell carcinoma by stabilizing CARM1. Biochem Biophys Res Commun 512:34–40. https://doi.org/10.1016/j.bbrc.2019.02.057
Qi H, Lu L, Wang L (2020) Long noncoding RNA ST7-AS1 upregulates TRPM7 expression by sponging microRNA-543 to promote cervical cancer progression. OncoTargets Ther 13:7257–7269. https://doi.org/10.2147/ott.s253868
Cai S, Weng Y, Liu P, Miao F (2020) Knockdown of ST7-AS1 inhibits migration, invasion, cell cycle progression and induces apoptosis of gastric cancer. Oncol Lett 19:777–782. https://doi.org/10.3892/ol.2019.11145
Ham IH, Lee D, Hur H (2019) Role of cancer-associated fibroblast in gastric cancer progression and resistance to treatments. J Oncol 2019:6270784. https://doi.org/10.1155/2019/6270784
Du B, Shim JS (2016) Targeting epithelial–mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. https://doi.org/10.3390/molecules21070965
Göllner S, Oellerich T, Agrawal-Singh S, Schenk T, Klein H-U, Rohde C, Pabst C, Sauer T, Lerdrup M, Tavor S, Stölzel F, Herold S, Ehninger G, Köhler G, Pan K-T, Urlaub H, Serve H, Dugas M, Spiekermann K, Vick B, Jeremias I, Berdel WE, Hansen K, Zelent A, Wickenhauser C, Müller LP, Thiede C, Müller-Tidow C (2017) Loss of the histone methyltransferase EZH2 induces resistance to multiple drugs in acute myeloid leukemia. Nat Med 23:69–78. https://doi.org/10.1038/nm.4247
Kikuchi J, Koyama D, Wada T, Izumi T, Hofgaard PO, Bogen B, Furukawa Y (2015) Phosphorylation-mediated EZH2 inactivation promotes drug resistance in multiple myeloma. J Clin Invest 125:4375–4390. https://doi.org/10.1172/jci80325
Hu B, Qin C, Li L, Wei L, Mo X, Fan H, Lei Y, Wei F, Zou D (2021) Midkine promotes glioblastoma progression via PI3K-Akt signaling. Cancer Cell Int 21:509. https://doi.org/10.1186/s12935-021-02212-3
Sandra F, Harada H, Nakamura N, Ohishi M (2004) Midkine induced growth of ameloblastoma through MAPK and Akt pathways. Oral Oncol 40:274–280. https://doi.org/10.1016/j.oraloncology.2003.08.011
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG (2013) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13:714–726. https://doi.org/10.1038/nrc3599
Kharaishvili G, Simkova D, Bouchalova K, Gachechiladze M, Narsia N, Bouchal J (2014) The role of cancer-associated fibroblasts, solid stress and other microenvironmental factors in tumor progression and therapy resistance. Cancer Cell Int 14:41. https://doi.org/10.1186/1475-2867-14-41
Sakamoto K, Kadomatsu K (2012) Midkine in the pathology of cancer, neural disease, and inflammation. Pathol Int 62:445–455. https://doi.org/10.1111/j.1440-1827.2012.02815.x
Xu Y, Qu X, Zhang X, Luo Y, Zhang Y, Luo Y, Hou K, Liu Y (2009) Midkine positively regulates the proliferation of human gastric cancer cells. Cancer Lett 279:137–144. https://doi.org/10.1016/j.canlet.2009.01.024
Zhao S, Wang H, Nie Y, Mi Q, Chen X, Hou Y (2012) Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity. Cancer Immunol Immunother 61:1745–1753. https://doi.org/10.1007/s00262-012-1235-3
Xu YY, Mao XY, Song YX, Zhao F, Wang ZN, Zhang WX, Xu HM, Jin F (2012) Midkine confers Adriamycin resistance in human gastric cancer cells. Tumour Biol 33:1543–1548. https://doi.org/10.1007/s13277-012-0406-3
Wu D, Ma Z, Ma D, Li Q (2021) Long non-coding RNA maternally expressed gene 3 affects cell proliferation, apoptosis and migration by targeting the microRNA-9-5p/midkine axis and activating the phosphoinositide-dependent kinase/AKT pathway in hepatocellular carcinoma. Oncol Lett 21:345. https://doi.org/10.3892/ol.2021.12606
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196. https://doi.org/10.1038/nrm3758
Zm X, Xy W, Am W (2015) Periostin induces chemoresistance in colon cancer cells through activation of the PI 3 K/A kt/survivin pathway. Biotechnol Appl Biochem 62:401–406
Funding
This work was supported by General Projects of Hunan Natural Science Foundation (2019JJ40493).
Author information
Authors and Affiliations
Contributions
Guarantor: Z-JZ; concepts: Z-JZ, D-YF; design: D-YF; literature research: K-DY; clinical studies: YW; experimental studies: Q-LL; data acquisition: FZ; analysis: B-HL; manuscript preparation: YW; manuscript editing: Z-JZ; and manuscript review: Z-JZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were performed with the approval by the Research Ethics Committee of Xiangya Hospital of Central South University (Changsha, China).
Consent for publication
The informed consent obtained from study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, KD., Wang, Y., Zhang, F. et al. CAF-derived midkine promotes EMT and cisplatin resistance by upregulating lncRNA ST7-AS1 in gastric cancer. Mol Cell Biochem 477, 2493–2505 (2022). https://doi.org/10.1007/s11010-022-04436-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04436-x