Abstract
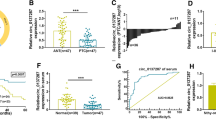
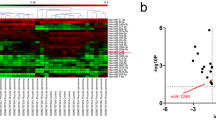
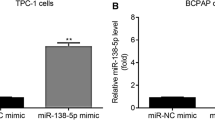
The aim of this study was to research the influences of miR-183-5p on the proliferation, invasion, and glycolysis of thyroid cancer (THCA) cells. Clinical specimens from 84 THCA patients were included. THCA cell lines (K1, SW1736, and TPC1) were cultured. siFOXO1, miR-183-5p mimic, or miR-183-5p inhibitors were transfected into THCA cells by Lipofectamine ™ 2000. qRT-PCR, western blot, and immunohistochemistry assays were used to detect miR-183-5p and FOXO1 expression. CCK-8 assay, colony formation, flow cytometry, Transwell, and wound healing experiment were utilized, respectively, to detect cell proliferation, colony formation, apoptosis, invasion, and migration. Glycolysis was evaluated by detecting glucose uptake, lactate production, ATP level, and glycolysis-related proteins expression. Dual-luciferase reporter assay and RNA pull-down assay were employed to verify the target relationship between miR-183-5p and FOXO1. The effect of miR-183-5p on THCA cells growth in vivo was researched using nude mice. miR-183-5p was highly expressed in THCA tissues and cells, correlating with poor outcome. miR-183-5p up-regulation attenuated apoptosis, and accelerated proliferation, colony formation, migration, invasion, and glycolysis of THCA cells. Opposite results were found by miR-183-5p down-regulation. FOXO1 was a target gene of miR-183-5p, where expression was directly inhibited by miR-183-5p. FOXO1 silencing reversed the inhibitory effect of miR-183-5p inhibitor on THCA cells malignant phenotype. miR-183-5p down-regulation inhibited THCA cells growth in vivo. miR-183-5p accelerates progression and glycolysis of THCA by targeting FOXO1. miR-183-5p was a novel target for THCA treatment.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Brooks E, Simmons-Arnold L, Naud S, Evans MF, Elhosseiny A (2009) Multinucleated giant cells’ incidence, immune markers, and significance: a study of 172 cases of papillary thyroid carcinoma. Head Neck Pathol 3:95–99
Kitahara CM, Sosa JA The changing incidence of thyroid cancer. Nat Rev Endocrinol
Liu S, Yang L, Yuan Y, Li H, Tian J, Lu S, Wang N, Ji J (2018) Cancer incidence in Beijing, 2014. Chin J Cancer Res 30:13
Khachatrian AS (2012) [Problem of early intra- and preoperative pathomorphologic diagnosis of thyroid cancer].11–16
Akçay G, Uslu H, Varoglu E, Tekin SB, Gündogdu C (1997) Assessment of thyroid nodules by technetium-99m-tetrofosmin scintigraphy. Br J Clin Pract 51:5–7
Rizvi AA and Selgrath C (1981) Fine-needle aspiration biopsy in the management of thyroid nodules. 134:198
Salehi M and Sharifi M (2018) Exosomal miRNAs as novel cancer biomarkers: challenges and opportunities. J Cell Physiol 233
Yip L, Kelly L, Shuai Y, Armstrong MJ, Nikiforov YE, Carty SE, Nikiforova MN MicroRNA signature distinguishes the degree of aggressiveness of papillary thyroid carcinoma. 18:2035–2041
Ashburner MBC, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene Ontology: tool for the unification of biology.The Gene Ontology Consortium. Nat Genet 25:25–29
Tetzlaff MT, Liu A, Xu X, Master SR, Baldwin DA, Tobias JW, Livolsi VA and Baloch ZW Differential expression of miRNAs in papillary thyroid carcinoma compared to multinodular goiter using formalin fixed paraffin embedded tissues. 18:163–173.
Sun M, Fang S, Li W, Li C, Wang Y (2015) Associations of miR-146a and miR-146b expression and clinical characteristics in papillary thyroid carcinoma. Cancer Biomark 15:33–40
Gao JM, Huang L-Z, Huang Z-G, He R-Q Clinical value and potential pathways of miR-183–5p in bladder cancer: A study based on miRNA-seq data and bioinformatics analysis. Oncol Lett
H Wang, Z Ma, X Liu, C Zhang, Y Hu MiR-183–5p is required for non-small cell lung cancer progression by repressing PTEN
Wang Z, Xia F, Feng T, Jiang B, Wang W, Li X (2020) OTUD6B-AS1 inhibits viability, migration, and invasion of thyroid carcinoma by targeting miR-183-5p and miR-21. Front Endocrinol 11:136. https://doi.org/10.3389/fendo.2020.00136
Ma J, Kan Z (2021) Circ_0137287 suppresses cell tumroigenesis and aerobic glycolysis in papillary thyroid carcinoma through miR-183-5p/PPP2R2A axis. Cytotechnology 73:497–511. https://doi.org/10.1007/s10616-021-00473-4
Feng Z, Chen R, Huang N, Luo C (2020) Long non-coding RNA ASMTL-AS1 inhibits tumor growth and glycolysis by regulating the miR-93-3p/miR-660/FOXO1 axis in papillary thyroid carcinoma. Life Sci 244:117298. https://doi.org/10.1016/j.lfs.2020.117298
Zheng M, Cao MX, Luo XJ, Li L, Wang K, Wang SS (2019) EZH2 promotes invasion and tumour glycolysis by regulating STAT3 and FoxO1 signalling in human OSCC cells. J Cell Mol Med 23:6942–6954
Srivastava LM, Hübscher G (1966) Glucose metabolism in the mucosa of the small intestine. Glycolysis in subcellular preparations from the cat and rat. Biochem J 100:458–466
Schwab A, Siddiqui A, Vazakidou ME, Napoli F, Ceppi P (2018) Polyol Pathway links glucose metabolism to the aggressiveness of cancer cells. Cancer Res 78:canres.2834.2017
Lodewijk L, Pins AM, Kist JW, Valk GD, Kranenburg O, Borel Rinkes IHM, Vriens MR (2012) The value of miRNA in diagnosing thyroid cancer: a systematic review. Cancer Biomark 11:229–238
Waseem M, Ahmad MK, Serajuddin M, Bhaskar V, Sankhwar SN, Mahdi AA MicroRNA-183–5p: a new potential marker for prostate cancer. Indian J Clin Biochem
Bi D-P, Yin C-H, Zhang X-Y, Yang N-N and Xu J-Y miR-183 functions as an oncogene by targeting ABCA1 in colon cancer. Oncol Rep
Yang X, Wang L, Wang Q, Li L, Fu Y and Sun J MiR-183 inhibits osteosarcoma cell growth and invasion by regulating LRP6-Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun S0006291X18301931
Wang W, Liu H, Wang S, Hao X, Li L (2011) A diterpenoid derivative 15-oxospiramilactone inhibits Wnt/β-catenin signaling and colon cancer cell tumorigenesis. Cell Res 21:730–740
Helmbrecht K, Kispert A, R. Von Wasielewski, Brabant G (2001) Identification of a Wnt/β-catenin signaling pathway in human thyroid cells. Endocrinology: https://doi.org/10.1210/endo.142.12.8554
Yamashita S, Meirmanov S, Sekine I, Nakashima M, Ito M, Saenko SV, Namba H, Abrosimov AA, Lantsov D and Naruke Y (2010) Cyclin D1 overexpression in thyroid papillary microcarcinoma: its association with tumour size and aberrant beta-catenin expression. 47:248–256.
Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J, Qin Y, Sun Z, Zheng X miR-183 inhibits TGF-β1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. 10:354–360.
Pennelli G, Galuppini F, Barollo S, Cavedon E, Bertazza L, Fassan M, Guzzardo V, Pelizzo MR, Rugge M, Mian C (2015) The PDCD4/miR-21 pathway in medullary thyroid carcinoma. Human Pathol 46:50–57
Gheysarzadeh A, Yazdanparast R Inhibition of H2O2-induced cell death through FOXO1 modulation by EUK-172 in SK-N-MC cells. Eur J Pharmacol 697
Hasegawa K, Kawahara T, Fujiwara K, Shimpuku M, Sasaki T, Kitamura T, Yoshikawa K (2012) Necdin controls foxo1 acetylation in hypothalamic arcuate neurons to modulate the thyroid axis. J Neurosc 32:5562–5572
Zaballos MA, Santisteban P (2013) FOXO1 controls thyroid cell proliferation in response to TSH and IGF-I and is involved in thyroid tumorigenesis. Mol Endocrinol 27:50–62
Song HM, Luo Y, Li DF, Wei CK, Fang L (2015) MicroRNA-96 plays an oncogenic role by targeting FOXO1 and regulating AKT/FOXO1/Bim pathway in papillary thyroid carcinoma cells. Int J Clin Exp Pathol 8:9889–9900
Yukari IK, Tsutomu S, Masaki K, Hye-Jin K, Yong-Soo L, Osamu K, Hiromi Y-H, Katsumi I, Domenico A, Tadahiro K (2012) Hepatic FoxO1 integrates glucose utilization and lipid synthesis through regulation of chrebp O-glycosylation. PLoS ONE 7:e47231
G.K. S (2005) limma: linear models for microarray data. bioinformatics and computational biology solutions using R and bioconductor. Statis Biol Health. https://doi.org/10.1007/0-387-29362-0_23
Feng C, Gao Y, Wang C, Yu X, Zhang W, Guan H, Shan Z, Teng W (2013) Aberrant overexpression of pyruvate kinase M2 is associated with aggressive tumor features and the\\r BRAF\\r mutation in papillary thyroid cancer. J Clin Endocrinol Metab 98:E1524–E1533
Nahm JH, Kim HM, Koo JS (2017) Glycolysis-related protein expression in thyroid cancer. Tumor Biol 10:1177101042831769600
Long W, Gong X, Yang Y, Yang K (2020) Downregulation of PER2 promotes tumor progression by enhancing glycolysis via the phosphatidylinositol 3-kinase/protein kinase B pathway in oral squamous cell carcinoma. J Oral Maxillofac Surg 78:1780.e1-1780.e14. https://doi.org/10.1016/j.joms.2020.05.035
Funding
The study is supported by Zhejiang Medical Science and Technology Project (2019KY331, 2020KY464, 2021KY086).
Author information
Authors and Affiliations
Contributions
CH, LT and KW conceived and designed the experiments, CH and LJ analyzed and interpreted the results of the experiments, CH and KM performed the experiments; all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All animal experiments have been approved by the animal ethics committee of First Affiliated Hospital, School of Medicine, Zhejiang University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, C., Mo, K., Jiang, L. et al. miR-183-5p promotes proliferation, invasion, and glycolysis of thyroid carcinoma cells by targeting FOXO1. Mol Cell Biochem 477, 1195–1206 (2022). https://doi.org/10.1007/s11010-022-04357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04357-9