Abstract
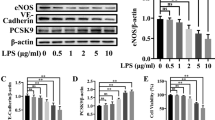
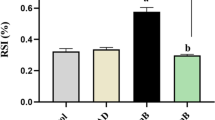
Edema is common in preeclampsia (preE), a hypertensive disorder of pregnancy. Cardiotonic steroids (CTSs) such as marinobufagenin (MBG) are involved in the pathogenesis of preE. To assess whether CTSs are involved in the leakage of lymphatic endothelial cell (LEC), we evaluated their effect on monolayer permeability of LECs (MPLEC) in culture. A rat mesenteric LECs were treated with DMSO (vehicle), and CTSs (MBG, CINO, OUB) at concentrations of 1, 10, and 100 nM. Some LECs were pretreated with 1 μM L-NAME (N-Nitro-l-Arginine Methyl Ester) before adding 100 nM MBG or cinobufotalin (CINO). Expression of β-catenin and vascular endothelial (VE)-cadherin in CTS-treated LECs was measured by immunofluorescence and MPLEC was quantified using a fluorescence plate reader. Western blot was performed to measure β-catenin and VE-cadherin protein levels and myosin light chain 20 (MLC20) phosphorylation. MBG (≥ 1 nM) and CINO (≥ 10 nM) caused an increase (p < 0.05) in the MPLEC compared to DMSO while ouabain (OUB) had no effect. Pretreatment of LECs with 1 μM L-NAME attenuated (p < 0.05) the MPLEC. The β-catenin expression in LECs was downregulated (p < 0.05) by MBG and CINO. However, there was no effect on the LECs tight junctions for the CINO group. VE-cadherin expression was downregulated (p < 0.05) by CINO, and MLC20 phosphorylation was upregulated (p < 0.05) by MBG. We demonstrated that MBG and CINO caused an increase in the MPLEC, which were attenuated by L-NAME pretreatment. The data suggest that CTSs exert their effect via nitric-oxide-dependent signaling pathway and may be involved in vascular leak syndrome of LEC lining in preE.





Similar content being viewed by others
References
Bridenbaugh EA, Gashev AA, Zawieja DC (2003) Lymphatic muscle: a review of contractile function. Lymphat Res Biol 1:147–158 (Review)
Puschett JB (2006) The role of excessive volume expansion in the pathogenesis of preeclampsia. Med Hypotheses 67:1125–1132
Pridjian G, Puschett JB (2002) Preeclampsia. Part 1: clinical and pathophysiologic considerations. Obstet Gynecol Surv 57(9):598–618 (Review)
Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC (2013) The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis 7:395–406
Uddin MN, Horvat D, Childs EW, Puschett JB (2009) Marinobufagenin causes endothelial cell monolayer hyperpermeability by altering apoptotic signaling. Am J Physiol Regul Integr Comp Physiol 296:R1726–R1734
Pridjian G, Puschett JB (2002) Preeclampsia. Part 2: experimental and genetic considerations. Obstet Gynecol Surv. 57:619–640
Vu H, Ianosi-Irimie MR, Pridjian C, Whitbred JM, Durst JM, Bagrov AY, Fedorova OV, Pridjian G, Puschett JB (2005) The involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol 25:520–528
Agunanne E, Horvat D, Harrison R, Uddin MN, Jones R, Kuehl TJ, Abi-Ghanem D, Berghman LR, Lai X, Li J, Romo D, Puschett JB (2011) Marinobufagenin levels in preeclamptic patients: a preliminary report. Am J Perinatol. 28:509–514
Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, Bagrov AY (1999) Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens 17:1179–1187
Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Müller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J (2013) Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123:2803–2815
Delacrétaz E, de Quay N, Waeber B, Vial Y, Schulz PE, Burnier M, Brunner HR, Bossart H, Schaad NC (1995) Differential nitric oxide synthase activity in human platelets during normal pregnancy and pre-eclampsia. Clin Sci (Lond) 88:607–610
Podjarny E, Losonczy G, Baylis C (2004) Animal models of preeclampsia. Semin Nephrol 24:596–606
Greenberg SS, Lancaster JR, Xie J, Sarphie TG, Zhao X, Hua L, Freeman T, Kapusta DR, Giles TD, Powers DR (1997) Effects of NO synthase inhibitors, arginine-deficient diet, and amiloride in pregnant rats. Am J Physiol 273:R1031–R1045
Uddin MN, McLean LB, Hunter FA, Horvat D, Severson J, Tharakan B, Childs EW, Puschett JB (2009) Vascular leak in a rat model of preeclampsia. Am J Nephrol 30:26–33
Uddin MN, Horvat D, Glaser SS, Danchuk S, Mitchell BM, Sullivan DE, Morris CA, Puschett JB (2008) Marinobufagenin inhibits proliferation and migration of cytotrophoblast and CHO cells. Placenta 29:266–273
Uddin MN, Horvat D, Glaser SS, Mitchell BM, Puschett JB (2008) Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J Biol Chem 283:17946–17953
Horvat D, Allen SR, Jones RO, Zawieja DC, Kuehl TJ, Uddin MN (2012) Cardiotonic steroids trigger cytotrophoblast dysfunction via cell cycle arrest and apoptotic signaling. J Investig Med 60:19106
Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X (2007) Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol 292:H3179–H3189
Ehrig J, Horvat D, Fothergill RE, Allen SR, Jones RO, Zawieja DC, Kuehl TJ, Uddin MN (2013) Cardiotonic steroids induce an anti-angiogenic profile in first trimester cytotrophoblast cells. Am J Obstet Gynecol 208(1):S99
Durán WN, Beuve AV, Sánchez FA (2013) Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB Life 65:819–826
Sánchez FA, Rana R, González FG, Iwahashi T, Durán RG, Fulton DJ, Beuve AV, Kim DD, Durán WN (2011) Functional significance of cytosolic endothelial nitric-oxide synthase (eNOS): regulation of hyperpermeability. J Biol Chem 286:30409–30414
Uddin MN, Agunanne E, Horvat D, Puschett JB (2009) Marinobufogenin causes enhanced permeability in human brain microvascular endothelial cells via apoptotic signaling. J Am Soc Nephrol 20:534A
Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M (2009) Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297:H726–H734
Moore TM, Norwood NR, Creighton JR, Babal P, Brough GH, Shasby DM, Stevens T (2000) Receptor-dependent activation of store-operated calcium entry increases endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 279:L691–L698
Tharakan B, Hellman J, Sawant DA, Tinsley JH, Parrish AR, Hunter FA, Smythe WR, Childs EW (2012) β-Catenin dynamics in the regulation of microvascular endothelial cell hyperpermeability. Shock 37:306–311
Wallez Y, Huber P (2007) Endothelial adherens and tight junctions in vascular homeostasis, inflammation, and angiogenesis. Biochim et Biophys Acta (BBA). 1778(3):794–809
Gory-Fauré S, Prandini M-H, Pointu H et al (1999) Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126:2093
Kai S, Lu J-H, Hui P-P, Zhao H (2014) Pre-clinical evaluation of cinobufotalin as a potential anti-lung cancer agent. Biochem Biophys Res Commun 452:768–774
Acknowledgements
Funding for this work was provided by Scott, Sherwood and Brindley Foundation and Department of Obstetrics and Gynecology (MNU) and the Noble Centennial Endowment for Research in Obstetrics and Gynecology (TJK), Baylor Scott & White Healthcare, Temple, Texas. The CTB cell line Sw-71 was kindly provided by Dr. Gil G. Mor at Yale University School of Medicine, New Haven, CT, USA. MBG was a kind gift from Drs. Alexei Y. Bagrov, Edward G. Lakatta, and Olga V. Fedorova at the National Institute on Aging (NIA), Baltimore, Maryland. Authors acknowledge the assistance of the Texas A&M University College of Medicine, Integrated Microscopy/Imaging Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horvat, D., Afroze, S.H., Cromer, W.E. et al. Cartiotonic steroids affect monolayer permeability in lymphatic endothelial cells. Mol Cell Biochem 476, 3207–3213 (2021). https://doi.org/10.1007/s11010-021-04147-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04147-9