Abstract
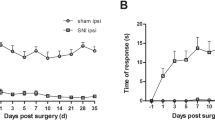
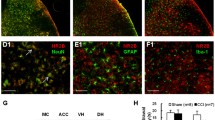
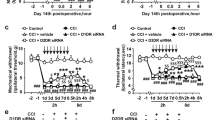
Neuropathic pain (NP) is chronic, intractable, and typically not alleviated using analgesics. Ferroptosis is a new type of cell death characterized by mitochondrial damage, oxidative stress, and mitochondrial dysfunction, affecting specific types of synaptic plasticity in the spinal cord. Here, we evaluated the role of ferroptosis in NP using chronic contractile injury (CCI) in rats. The CCI and control groups were subjected to sciatic nerve ligation. The mechanical withdrawal threshold and thermal withdrawal reflex latency were used to detect changes in mechanical pain threshold and thermal pain threshold in rats, respectively. Notably, CCI caused mechanical and thermal stimulation of the injured hind paw, reduced levels of glutathione peroxidase 4 (GPX4), and increased acyl-CoA synthetase long-chain family member 4 (ACSL4). Treatment with the ferroptosis inhibitor ferrostatin-1 (10 mg/kg) 1 h after surgery upregulated GPX4 expression and downregulated ACSL4 expression, whereas the ferroptosis inducer, erastin (10 mg/kg), exerted opposite effects. Treatment with ferrostatin-1 upregulated NeuN expression and downregulated GPX4 expression, whereas erastin reversed these effects. CCI increased the number of damaged mitochondria and decreased the mean planar mitochondrial area, and treatment with erastin further exacerbated these effects. The iron ion content in the spinal cords of CCI-induced rats increased. Treatment with ferrostatin-1 decreased, whereas treatment with erastin increased iron ion content in the CCI-induced rat model. Taken together, our results showed that ferroptosis is involved in the development of NP in male rats by blocking neuron and astrocyte activation in the spinal dorsal horn.








Similar content being viewed by others
Data availability
If necessary, contact the corresponding author to obtain the data.
References
Baad-Hansen L, Benoliel R (2017) Neuropathic orofacial pain: facts and fiction. Cephalalgia 37(7):670–679
Nadine A (2012) Neuropathic pain: mechanisms, therapeutic approach, and interpretation of clinical trials. Continuum (Minneap Minn) 32(2):512–532
Meacham K, Shepherd A, Mohapatra DP, Haroutounian S (2017) Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Head Rep 21(6):28–34
Kramer K, Minhas NK, Jutzeler CR, Erskine ELKS, Liu L, Ramer MS (2017) Neuropathic pain following traumatic spinal cord injury: models, measurement, and mechanisms. J Neurosci Res 95(6):1295–1306
Didangelos T, Doupis J, Veves A (2014) Painful diabetic neuropathy: clinical aspects. Handb Clin Neurol 126(126):53–61
Hatch MN, Cushing TR, Carlson GD, Chang EY (2017) Neuropathic pain and sci: identification and treatment strategies in the 21st century. J Neurol Sci 384:75–83
Jensen TS, Finnerup NB (2014) Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 13(9):924–935
Gierthmuhlen J, Baron R (2016) Neuropathic pain. Semin Neurol 36(5):462–468
Gilron I, Baron R, Jensen T (2015) Neuropathic pain: principles of diagnosis and treatment. Mayo Clinic Proc 90(4):532–545
Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ (2010) Nadph oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci 107(33):14851–14856
Dixon SJ, Lemberg K (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Li Q, Han X, Lan X et al (2017) Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 7:77
Fanzani A, Poli M (2017) Iron oxidative damage and ferroptosis in rhabdomyosarcoma. Int J Mol Sci 18(8):1718
Forcina GC, Dixon SJ (2019) GPX4 at the crossroads of lipid. Homeo Ferrop Proteom 19(18):e1800311
Müller T, Dewitz C et al (2017) Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci 74(19):3631–3645
Sebastian DBP, Tyurina YY, Panzilius E et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13:91–98
Kenny EM, Fidan E et al (2019) Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit Care Med 47(3):410–418
Latunde-Dada GO (2017) Ferroptosis: Role of lipid peroxidation iron and ferritinophagy. Biochim Biophys Acta 1861(8):1893–1900
Charan J, Kantharia ND (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4(4):303–306. https://doi.org/10.4103/0976-500X.119726.PMID:24250214;PMCID:PMC3826013
National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press (US), Washington (DC)
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Hamasaki M, Shibutani ST, Yoshimori T (2013) Up-to-date membrane biogenesis in the autophagosome formation. Curr Opin Cell Biol 25(4):455–460
Gregory NS, Harris AL et al (2019) An overview of animal models of pain: disease models and outcome measures. J Pain 14(11):1255–1269
Rasulic L, Singh V, Gopalakrishnan MS et al (2019) Neuropathic pain: searching for the magic bullet. Neurol India 67(Supplement):S25–S26
Singh JA, Jain V, Singh N (2011) Animal models of neuropathic pain: animal models of neuropathic pain. Methods Mol Med 25(1):1–28
Kumar A, Kaur H, Singh A (2018) Neuropathic pain models caused by damage to central or peripheral nervous system. Pharmacol Rep 70(2):206–216
Hamasaki M, Shibutani ST, Yoshimori T (2013) Up-to-date membrane biogenesis in the autophagosome formation. Curr Opin Cell Biol 25(4):455–460
Widerström-Noga E (2017) Neuropathic pain and spinal cord injury: phenotypes and pharmacological management. Drugs 77(9):967–984
van Hecke ASK, Khan RA, Smith BH, Torrance N (2014) Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155(4):654–662
Xie J, Liu S, Wu B (2016) The protective effect of resveratrol in the transmission of neuropathic pain mediated by the P2X7 receptor in the dorsal root ganglia. Neurochem Int 103:24–35
Finnerup NB (2017) Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord 55(12):1046–1050
Doll S, Proneth B, Tyurina YY (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13(1):91–98
Kenny EM, Fidan E, Yang Q (2019) Ferroptosis contributes to neuronal death and functional outcome after traumatic. Brain Injury Crit Care Med 47(3):410–418
Maiorino M, Conrad M, Ursini F (2018) GPx4 lipid peroxidation and cell death: discoveries rediscoveries and open issues. Antioxid Redox Signal 29(1):61–74
Yuan H, Li X, Zhang X, Kang R, Tang D (2016) Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun 478(3):1338–1343
Geng N, Shi BJ, Li SL et al (2018) Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci 22(12):3826–3836
Guo D, Hu X, Zhang H, Lu C, Cui G, Luo X (2018) Orientin and neuropathic pain in rats with spinal nerve ligation. Int Immunopharmacol 58:72–79
Saleem M, Deal B, Nehl E, Janjic JM, Pollock JA (2019) Nanomedicine-driven neuropathic pain relief in a rat model is associated with macrophage polarity and mast cell activation. Acta Neuropathol Commun 7(1):108–116
Langley PC, Van Litsenburg C, Cappelleri JC, Carroll D (2013) The burden associated with neuropathic pain in Western Europe. J Med Econ 16(1):85–95
Mosley GE, Wang M, Nasser P, Lai A, Charen DA, Zhang B, Iatridis JC (2020) Males and females exhibit distinct relationships between intervertebral disc degeneration and pain in a rat model. Sci Rep 10(1):15120
Gervais JA, Otis C, Lussier B, Guillot M, Martel-Pelletier J, Pelletier JP, Beaudry F, Troncy E (2019) Osteoarthritic pain model influences functional outcomes and spinal neuropeptidomics: a pilot study in female rats. Can J Vet Res 83(2):133–141
North RY, Li Y, Ray P, Rhines LD, Tatsui CE, Rao G, Johansson CA, Zhang H, Kim YH, Zhang B, Dussor G, Kim TH, Price TJ, Dougherty PM (2019) Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 142(5):1215–1226
Acknowledgements
We thank the Deputy Chief Physician, Jiang Ning, the Central Laboratory of the Second Hospital of Tianjin Medical University for assistance with experimental techniques.
Funding
Tianjin Natural Science Foundation supported this research project (Grant No. 18JCQNJC13100).
Author information
Authors and Affiliations
Contributions
HW, XH, and CH jointly participated in the experimental design, experimental operation, and writing. JN guided the design and writing. HC, BL, JL, and WM participated in the design and performed experiments. QL and YY performed experiments. KS provided experimental ideas and guided the writing of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Ethics approval
All procedures involved in this experiment were approved by the Animal Clinical Committee of Tianjin Medical University (Approval: DWLI-20181010) and were performed in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines (National Institutes of Health Institute Publication 85-23, revised in 1996).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Huo, X., Han, C. et al. Ferroptosis is involved in the development of neuropathic pain and allodynia. Mol Cell Biochem 476, 3149–3161 (2021). https://doi.org/10.1007/s11010-021-04138-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04138-w