Abstract
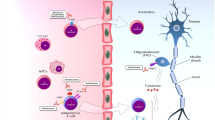
Multiple sclerosis (MS) is an autoimmune chronic inflammatory disease of the central nervous system with a wide range of symptoms, like executive function defect, cognitive dysfunction, blurred vision, decreased sensation, spasticity, fatigue, and other symptoms. This neurological disease is characterized by the destruction of the blood–brain barrier, loss of myelin, and damage to neurons. It is the result of immune cells crossing the blood–brain barrier into the central nervous system and attacking self-antigens. Heretofore, many treatments proved that they can retard the progression of the disease even though there is no cure. Therefore, treatments aimed at improving patients' quality of life and reducing adverse drug reactions and costs are essential. In this review, the treatment approaches to alleviate the progress of MS include the following: pharmacotherapy, antibody therapy, cell therapy, gene therapy, and surgery. The current treatment methods of MS are described in terms of the prevention of myelin shedding, the promotion of myelin regeneration, and the protection of neurons.
Similar content being viewed by others
References
Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL (2009) Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol 10(9):958–964. https://doi.org/10.1038/ni.1775
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8(9):913–919. https://doi.org/10.1038/ni1507
Hemmer B, Kerschensteiner M, Korn T (2015) Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 14(4):406–419. https://doi.org/10.1016/s1474-4422(14)70305-9
Comabella M, Khoury SJ (2012) Immunopathogenesis of multiple sclerosis. Clin Immunol 142(1):2–8. https://doi.org/10.1016/j.clim.2011.03.004
Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S (2015) Advances in the immunopathogenesis of multiple sclerosis. Curr Opin Neurol 28(3):206–219. https://doi.org/10.1097/WCO.0000000000000205
Lazibat I, Rubinic Majdak M, Zupanic S (2018) Multiple sclerosis: new aspects of immuno-pathogenesis. Acta Clin Croat 57(2):352–361. https://doi.org/10.20471/acc.2018.57.02.17
Sorbara CD, Wagner NE, Ladwig A, Nikic I, Merkler D, Kleele T, Marinkovic P, Naumann R, Godinho L, Bareyre FM, Bishop D, Misgeld T, Kerschensteiner M (2014) Pervasive axonal transport deficits in multiple sclerosis models. Neuron 84(6):1183–1190. https://doi.org/10.1016/j.neuron.2014.11.006
Feinstein A, Freeman J, Lo AC (2015) Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol 14(2):194–207. https://doi.org/10.1016/s1474-4422(14)70231-5
Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner I-K, Rovira A, Sumowski JF, Valsasina P, Filippi M (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14(3):302–317. https://doi.org/10.1016/s1474-4422(14)70250-9
Williams S (2014) In the clinic: multiple sclerosis. Ann Intern Med 160(7):ITC4. https://doi.org/10.7326/0003-4819-160-7-201404010-01004
Chitnis T, Arnold DL, Banwell B, Bruck W, Ghezzi A, Giovannoni G, Greenberg B, Krupp L, Rostasy K, Tardieu M, Waubant E, Wolinsky JS, Bar-Or A, Stites T, Chen Y, Putzki N, Merschhemke M, Gartner J, Group PS (2018) Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med 379(11):1017–1027. https://doi.org/10.1056/NEJMoa1800149
Newsome SD, Mokliatchouk O, Castrillo-Viguera C, Naylor ML (2020) Matching-adjusted comparisons demonstrate better clinical outcomes in patients with relapsing multiple sclerosis treated with peginterferon beta-1a than with teriflunomide. Mult Scler Relat Disord 40:101954. https://doi.org/10.1016/j.msard.2020.101954
Sellebjerg F, Hedegaard CJ, Krakauer M, Hesse D, Lund H, Nielsen CH, Sondergaard HB, Sorensen PS (2012) Glatiramer acetate antibodies, gene expression and disease activity in multiple sclerosis. Mult Scler 18(3):305–313. https://doi.org/10.1177/1352458511420268
Kappos L, Bar-Or A, Cree BAC et al (2018) Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. The Lancet 391(10127):1263–1273. https://doi.org/10.1016/s0140-6736(18)30475-6
Hartung H-P, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, Krapf H, Zwingers T (2002) Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. The Lancet 360(9350):2018–2025. https://doi.org/10.1016/s0140-6736(02)12023-x
Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, Preiningerova J, Rizzo M, Singh I (2004) Oral simvastatin treatment in relapsing-remitting multiple sclerosis. The Lancet 363(9421):1607–1608. https://doi.org/10.1016/s0140-6736(04)16205-3
Kalincik T, Kubala Havrdova E, Horakova D, Izquierdo G, Prat A, Girard M, Duquette P, Grammond P, Onofrj M, Lugaresi A, Ozakbas S, Kappos L, Kuhle J, Terzi M, Lechner-Scott J, Boz C, Grand’Maison F, Prevost J, Sola P, Ferraro D, Granella F, Trojano M, Bergamaschi R, Pucci E, Turkoglu R, McCombe PA, Pesch VV, Van Wijmeersch B, Solaro C, Ramo-Tello C, Slee M, Alroughani R, Yamout B, Shaygannejad V, Spitaleri D, Sanchez-Menoyo JL, Ampapa R, Hodgkinson S, Karabudak R, Butler E, Vucic S, Jokubaitis V, Spelman T, Butzkueven H (2019) Comparison of fingolimod, dimethyl fumarate and teriflunomide for multiple sclerosis. J Neurol Neurosurg Psychiatry 90(4):458–468. https://doi.org/10.1136/jnnp-2018-319831
Miller AE (2017) Oral teriflunomide in the treatment of relapsing forms of multiple sclerosis: clinical evidence and long-term experience. Ther Adv Neurol Disord 10(12):381–396. https://doi.org/10.1177/1756285617722500
Polman CH (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365(14):1293–1303. https://doi.org/10.1056/NEJMoa1014656
Lindsey JW, Haden-Pinneri K, Memon NB, Buja LM (2012) Sudden unexpected death on fingolimod. Mult Scler 18(10):1507–1508. https://doi.org/10.1177/1352458512438456
Marriott JJ, Miyasaki JM, Gronseth G, O’Connor PW, Therapeutics, Technology Assessment Subcommittee of the American Academy of N (2010) Evidence report: the efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 74(18):1463–1470. https://doi.org/10.1212/WNL.0b013e3181dc1ae0
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT, Investigators DS (2012) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367(12):1098–1107. https://doi.org/10.1056/NEJMoa1114287
Hassan-Smith G, Douglas MR (2011) Management and prognosis of multiple sclerosis. Br J Hosp Med 72(11):174–176. https://doi.org/10.12968/hmed.2011.72.sup11.m174
Tourbah A (2015) Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology 110(Pt B):644–653. https://doi.org/10.1016/j.neuropharm.2015.08.028
Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, De Seze J, Debouverie M, Gout O, Clavelou P, Defer G, Laplaud DA, Moreau T, Labauge P, Brochet B, Sedel F, Pelletier J, group M-Ss (2016) MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult Scler 22(13):1719–1731. https://doi.org/10.1177/1352458516667568
Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL (2013) A regenerative approach to the treatment of multiple sclerosis. Nature 502(7471):327–332. https://doi.org/10.1038/nature12647
Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, Inman J, Arnow S, Devereux M, Abounasr A, Nobuta H, Zhu A, Friessen M, Gerona R, von Büdingen HC, Henry RG, Hauser SL, Chan JR (2017) Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. The Lancet 390(10111):2481–2489. https://doi.org/10.1016/s0140-6736(17)32346-2
Schwartzbach CJ, Grove RA, Brown R, Tompson D, Then Bergh F, Arnold DL (2017) Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing-remitting multiple sclerosis: a randomised, single-blind, phase II study. J Neurol 264(2):304–315. https://doi.org/10.1007/s00415-016-8341-7
Calic Z, Cappelen-Smith C, Hodgkinson SJ, McDougall A, Cuganesan R, Brew BJ (2015) Treatment of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome with intravenous immunoglobulin in a patient with multiple sclerosis treated with fingolimod after discontinuation of natalizumab. J Clin Neurosci 22(3):598–600. https://doi.org/10.1016/j.jocn.2014.08.016
Oconnor P (2015) A randomized trial of teriflunomide added to glatiramer acetate in relapsing multiple sclerosis. Mult Scler J Exp Transl Clin 1:1–10. https://doi.org/10.1177/2055217315618687
Krishnan AV, Kiernan MC, Huynh W, Arnold R (2015) Ion channel modulation as a therapeutic approach in multiple sclerosis. Curr Med Chem 22(38):4366–4378. https://doi.org/10.2174/0929867322666151029104452
Waxman SG (2008) Mechanisms of disease: sodium channels and neuroprotection in multiple sclerosis—current status. Nat Clin Pract Neurol 4(3):159–169. https://doi.org/10.1038/ncpneuro0735
Silva RBM, Greggio S, Venturin GT, da Costa JC, Gomez MV, Campos MM (2018) Beneficial effects of the calcium channel blocker CTK 01512–2 in a mouse model of multiple sclerosis. Mol Neurobiol 55(12):9307–9327. https://doi.org/10.1007/s12035-018-1049-1
Naziroglu M, Kutluhan S, Övey İS, Aykur M, Yurekli VA (2013) Modulation of oxidative stress, apoptosis, and calcium entry in leukocytes of patients with multiple sclerosis by Hypericum perforatum. Nutr Neurosci 17(5):214–221. https://doi.org/10.1179/1476830513y.0000000083
Raftopoulos R, Hickman SJ, Toosy A, Sharrack B, Mallik S, Paling D, Altmann DR, Yiannakas MC, Malladi P, Sheridan R, Sarrigiannis PG, Hoggard N, Koltzenburg M, Gandini Wheeler-Kingshott CAM, Schmierer K, Giovannoni G, Miller DH, Kapoor R (2016) Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 15(3):259–269. https://doi.org/10.1016/s1474-4422(16)00004-1
Landi D, Albanese M, Buttari F, Monteleone F, Boffa L, Rossi S, Motta C, Puma E, Centonze D (2017) Management of flu-like syndrome with cetirizine in patients with relapsing-remitting multiple sclerosis during therapy with interferon beta: results of a randomized, cross-over, placebo-controlled pilot study. PLoS ONE 12(7):e0165415. https://doi.org/10.1371/journal.pone.0165415
Markowitz CE (2007) Interferon-beta mechanism of action and dosing issues. Neurology 68(24 Suppl 4):S8-11. https://doi.org/10.1212/01.wnl.0000277703.74115.d2
Farina C, Weber MS, Meinl E, Wekerle H, Hohlfeld R (2016) Glatiramer acetate in multiple sclerosis: update on potential mechanisms of action. Lancet Neurol 4(9):567–575. https://doi.org/10.1016/S1474-4422(05)70167-8
Ruggieri M, Avolio C, Livrea P, Trojano M (2010) Glatiramer acetate in multiple sclerosis: a review. CNS Drug Rev 13(2):178–191. https://doi.org/10.1111/j.1527-3458.2007.00010.x
Longbrake EE, Hafler DA (2019) Siponimod chips away at progressive MS. Cell 179(7):1440. https://doi.org/10.1016/j.cell.2019.11.034
Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362(5):402–415. https://doi.org/10.1056/NEJMoa0907839
Neuhaus O, Strasser-Fuchs S, Fazekas F, Kieseier BC, Niederwieser G, Hartung HP, Archelos JJ (2002) Statins as immunomodulators comparison with interferon-1b in MS. Neurology 59(7):990–997. https://doi.org/10.1212/wnl.59.7.990
Kapoor R, Furby J, Hayton T, Smith KJ, Altmann DR, Brenner R, Chataway J, Hughes RAC, Miller DH (2010) Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol 9(7):681–688. https://doi.org/10.1016/s1474-4422(10)70131-9
Bartollino S, Chiosi F, di Staso S, Uva M, Pascotto A, Rinaldi M, Hesselink JMK, Costagliola C (2018) The retinoprotective role of phenytoin. Drug Des Dev Ther 12:3485–3489. https://doi.org/10.2147/DDDT.S169621
Herwerth M, Hemmer B (2017) Daclizumab for the treatment of relapsing-remitting multiple sclerosis. Expert Opin Biol Ther 17(6):747–753. https://doi.org/10.1080/14712598.2017.1304913
Naegelin Y, Naegelin P, von Felten S, Lorscheider J, Sonder J, Uitdehaag BMJ, Scotti B, Zecca C, Gobbi C, Kappos L, Derfuss T (2019) Association of rituximab treatment with disability progression among patients with secondary progressive multiple sclerosis. JAMA Neurol 76(3):274–281. https://doi.org/10.1001/jamaneurol.2018.4239
Evan JR, Bozkurt SB, Thomas NC, Bagnato F (2018) Alemtuzumab for the treatment of multiple sclerosis. Expert Opin Biol Ther 18(3):323–334. https://doi.org/10.1080/14712598.2018.1425388
Graf J, Aktas O, Rejdak K, Hartung HP (2019) Monoclonal antibodies for multiple sclerosis: an update. BioDrugs 33(1):61–78. https://doi.org/10.1007/s40259-018-0327-9
Myhr KM, Torkildsen O, Lossius A, Bo L, Holmoy T (2019) B cell depletion in the treatment of multiple sclerosis. Expert Opin Biol Ther 19(3):261–271. https://doi.org/10.1080/14712598.2019.1568407
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, Investigators OC (2017) Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 376(3):209–220. https://doi.org/10.1056/NEJMoa1606468
Devonshire V, Phillips R, Wass H, Da Roza G, Senior P (2018) Monitoring and management of autoimmunity in multiple sclerosis patients treated with alemtuzumab: practical recommendations. J Neurol 265(11):2494–2505. https://doi.org/10.1007/s00415-018-8822-y
Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, Frederiksen J, Skeen M, Jaffe GJ, Butzkueven H, Ziemssen F, Massacesi L, Chai Y, Xu L, Freeman S (2017) Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 16(3):189–199. https://doi.org/10.1016/s1474-4422(16)30377-5
Hanf KJM, Arndt JW, Liu Y, Gong BJ, Rushe M, Sopko R, Massol R, Smith B, Gao Y, Dalkilic-Liddle I, Lee X, Mojta S, Shao Z, Mi S, Pepinsky RB (2020) Functional activity of anti-LINGO-1 antibody opicinumab requires target engagement at a secondary binding site. mAbs 12(1):1713648. https://doi.org/10.1080/19420862.2020.1713648
Annunziata P, Masi G, Cioni C (2019) Association of circulating anti-CD64 IgM levels with favourable long-term clinical outcomes in multiple sclerosis patients. J Neuroimmunol 330:130–135. https://doi.org/10.1016/j.jneuroim.2019.03.005
Katsavos S, Coles A (2018) Alemtuzumab as treatment for multiple sclerosis. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a032029
Bergman J, Burman J, Gilthorpe JD, Zetterberg H, Jiltsova E, Bergenheim T, Svenningsson A (2018) Intrathecal treatment trial of rituximab in progressive MS: an open-label phase 1b study. Neurology 91(20):e1893–e1901. https://doi.org/10.1212/WNL.0000000000006500
Ryerson LZ, Foley J, Chang I, Kister I, Cutter G, Metzger RR, Goldberg JD, Li X, Riddle E, Smirnakis K, Kasliwal R, Ren Z, Hotermans C, Ho P-R, Campbell N (2019) Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology 93(15):e1452–e1462. https://doi.org/10.1212/wnl.0000000000008243
Willis MD, Hope-Gill B, Flood-Page P, Joseph F, Needham E, Jones J, Coles A, Robertson NP (2018) Sarcoidosis following alemtuzumab treatment for multiple sclerosis. Mult Scler 24(13):1779–1782. https://doi.org/10.1177/1352458518790391
Wingerchuk DM, Carter JL (2014) Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc 89(2):225–240. https://doi.org/10.1016/j.mayocp.2013.11.002
Pfeuffer S, Rolfes L, Bormann E, Sauerland C, Ruck T, Schilling M, Melzer N, Brand M, Pul R, Kleinschnitz C, Wiendl H, Meuth SG (2019) Comparing plasma exchange to escalated methyl prednisolone in refractory multiple sclerosis relapses. J Clin Med 9(1):35. https://doi.org/10.3390/jcm9010035
Dorst J, Fangerau T, Taranu D, Eichele P, Dreyhaupt J, Michels S, Schuster J, Ludolph AC, Senel M, Tumani H (2019) Safety and efficacy of immunoadsorption versus plasma exchange in steroid-refractory relapse of multiple sclerosis and clinically isolated syndrome: a randomised, parallel-group, controlled trial. EClinicalMedicine 16:98–106. https://doi.org/10.1016/j.eclinm.2019.10.017
Lipphardt M, Muhlhausen J, Kitze B, Heigl F, Mauch E, Helms HJ, Muller GA, Koziolek MJ (2019) Immunoadsorption or plasma exchange in steroid-refractory multiple sclerosis and neuromyelitis optica. J Clin Apher 34(4):381–391. https://doi.org/10.1002/jca.21686
Manguinao M, Krysko KM, Maddike S, Rutatangwa A, Francisco C, Hart J, Chong J, Graves JS, Waubant E (2019) A retrospective cohort study of plasma exchange in central nervous system demyelinating events in children. Mult Scler Relat Disord 35:50–54. https://doi.org/10.1016/j.msard.2019.07.004
Chegini A, Moghadami M, Maghari A (2020) Therapeutic plasma exchange in Tehran blood transfusion between 2011 and 2014. Ther Apher Dial 24(2):230–234. https://doi.org/10.1111/1744-9987.12864
Jamshidian A, Abd-Nikfarjam B, Khademi Z, Shaygannejad V, Salehi M (2020) Therapeutic plasma exchange may adjust IL-6 and TGF-beta signals in relapsed MS patients peripheral blood. J Clin Apher 35(2):72–78. https://doi.org/10.1002/jca.21755
Gravesteijn AS, Beckerman H, de Jong BA, Hulst HE, de Groot V (2020) Neuroprotective effects of exercise in people with progressive multiple sclerosis (Exercise PRO-MS): study protocol of a phase II trial. BMC Neurol 20(1):177. https://doi.org/10.1186/s12883-020-01765-6
Jiang P, Selvaraj V, Deng W (2010) Differentiation of embryonic stem cells into oligodendrocyte precursors. J Vis Exp 39:1960. https://doi.org/10.3791/1960
Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T (2008) Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS ONE 3(9):e3145. https://doi.org/10.1371/journal.pone.0003145
Shroff G (2016) Transplantation of human embryonic stem cells in patients with multiple sclerosis and lyme disease. Am J Case Rep 17:944–949. https://doi.org/10.12659/ajcr.899745
Massa MG, Gisevius B, Hirschberg S, Hinz L, Schmidt M, Gold R, Prochnow N, Haghikia A (2016) Multiple sclerosis patient-specific primary neurons differentiated from urinary renal epithelial cells via induced pluripotent stem cells. PLoS ONE 11(5):e0155274. https://doi.org/10.1371/journal.pone.0155274
Czepiel M, Balasubramaniyan V, Schaafsma W, Stancic M, Mikkers H, Huisman C, Boddeke E, Copray S (2011) Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia 59(6):882–892. https://doi.org/10.1002/glia.21159
Thiruvalluvan A, Czepiel M, Kap YA, Mantingh-Otter I, Vainchtein I, Kuipers J, Bijlard M, Baron W, Giepmans B, Bruck W, t Hart BA, Boddeke E, Copray S (2016) Survival and functionality of human induced pluripotent stem cell-derived oligodendrocytes in a nonhuman primate model for multiple sclerosis. Stem Cells Transl Med 5(11):1550–1561. https://doi.org/10.5966/sctm.2016-0024
Herszfeld D, Payne NL, Sylvain A, Sun G, Bernard CC, Clark J, Sathananthan H (2014) Fine structure of neurally differentiated iPS cells generated from a multiple sclerosis (MS) patient: a case study. Microsc Microanal 20(6):1869–1875. https://doi.org/10.1017/S1431927614013312
Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V (2014) Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep 3(2):250–259. https://doi.org/10.1016/j.stemcr.2014.06.012
Kojima K, Miyoshi H, Nagoshi N, Kohyama J, Itakura G, Kawabata S, Ozaki M, Iida T, Sugai K, Ito S, Fukuzawa R, Yasutake K, Renault-Mihara F, Shibata S, Matsumoto M, Nakamura M, Okano H (2019) Selective ablation of tumorigenic cells following human induced pluripotent stem cell-derived neural stem/progenitor cell transplantation in spinal cord injury. Stem Cells Transl Med 8(3):260–270. https://doi.org/10.1002/sctm.18-0096
Deng J, Zhang Y, Xie Y, Zhang L, Tang P (2018) Cell transplantation for spinal cord injury: tumorigenicity of induced pluripotent stem cell-derived neural stem/progenitor cells. Stem Cells Int 2018:5653787. https://doi.org/10.1155/2018/5653787
Ben-David U, Benvenisty N (2011) The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 11(4):268–277. https://doi.org/10.1038/nrc3034
Capuano R, Spitalieri P, Talarico RV, Catini A, Domakoski AC, Martinelli E, Scioli MG, Orlandi A, Cicconi R, Paolesse R, Novelli G, Di Natale C, Sangiuolo F (2018) Volatile compounds emission from teratogenic human pluripotent stem cells observed during their differentiation in vivo. Sci Rep 8(1):11056. https://doi.org/10.1038/s41598-018-29212-0
Shinde V, Perumal Srinivasan S, Henry M, Rotshteyn T, Hescheler J, Rahnenfuhrer J, Grinberg M, Meisig J, Bluthgen N, Waldmann T, Leist M, Hengstler JG, Sachinidis A (2016) Comparison of a teratogenic transcriptome-based predictive test based on human embryonic versus inducible pluripotent stem cells. Stem Cell Res Ther 7(1):190. https://doi.org/10.1186/s13287-016-0449-2
Zhao T, Zhang Z-n, Westenskow PD, Todorova D, Hu Z, Lin T, Rong Z, Kim J, He J, Wang M, Clegg Dennis O, Yang Y-g, Zhang K, Friedlander M, Xu Y (2015) Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell 17(3):353–359. https://doi.org/10.1016/j.stem.2015.07.021
Darlington PJ, Touil T, Doucet JS, Gaucher D, Zeidan J, Gauchat D, Corsini R, Kim HJ, Duddy M, Jalili F, Arbour N, Kebir H, Chen J, Arnold DL, Bowman M, Antel J, Prat A, Freedman MS, Atkins H, Sekaly R, Cheynier R, Bar-Or A, Canadian MSBMTSG (2013) Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann Neurol 73(3):341–354. https://doi.org/10.1002/ana.23784
Scolding NJ, Pasquini M, Reingold SC, Cohen JA, International Conference on Cell-Based Therapies for Multiple S, International Conference on Cell-Based Therapies for Multiple S, International Conference on Cell-Based Therapies for Multiple S (2017) Cell-based therapeutic strategies for multiple sclerosis. Brain 140(11):2776–2796. https://doi.org/10.1093/brain/awx154
Mariottini A, De Matteis E, Muraro PA (2020) Haematopoietic stem cell transplantation for multiple sclerosis: current status. BioDrugs 34(3):307–325. https://doi.org/10.1007/s40259-020-00414-1
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8(9):726–736. https://doi.org/10.1038/nri2395
Togha M, Jahanshahi M, Alizadeh L, Jahromi SR, Vakilzadeh G, Alipour B, Gorji A, Ghaemi A (2017) Rapamycin augments immunomodulatory properties of bone marrow-derived mesenchymal stem cells in experimental autoimmune encephalomyelitis. Mol Neurobiol 54(4):2445–2457. https://doi.org/10.1007/s12035-016-9840-3
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106(5):1755–1761. https://doi.org/10.1182/blood-2005-04-1496
Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH (2012) Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci 15(6):862–870. https://doi.org/10.1038/nn.3109
Mansoor SR, Zabihi E, Ghasemi-Kasman M (2019) The potential use of mesenchymal stem cells for the treatment of multiple sclerosis. Life Sci 235:116830. https://doi.org/10.1016/j.lfs.2019.116830
Grade S, Bernardino L, Malva JO (2013) Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies. Int J Dev Neurosci 31(7):692–700. https://doi.org/10.1016/j.ijdevneu.2013.01.004
Genc B, Bozan HR, Genc S, Genc K (2019) Stem cell therapy for multiple sclerosis. Adv Exp Med Biol 1084:145–174. https://doi.org/10.1007/5584_2018_247
Matsas R, Lavdas A, Papastefanaki F, Thomaidou D (2008) Schwann cell transplantation for CNS repair. Curr Med Chem 15(2):151–160. https://doi.org/10.2174/092986708783330593
Alamouti MA, Bakhtiyari M, Moradi F, Mokhtari T, Barbarestani M (2014) Remyelination of the corpus callosum by olfactory ensheathing cell in an experimental model of multiple sclerosis. Acta Med Iran 53(9):533–539
Li J, Chen W, Li YA, Chen Y, Zhang X (2015) Transplantation of olfactory ensheathing cells promotes partial recovery in rats with experimental autoimmune encephalomyelitis. Int J Clin Exp Pathol 8(9):11149–11156
Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G (2014) Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med 20(1):69–74. https://doi.org/10.1038/nm.3411
Wu C, Pot C, Apetoh L, Thalhamer T, Zhu B, Murugaiyan G, Xiao S, Lee Y, Rangachari M, Yosef N, Kuchroo VK (2013) Metallothioneins negatively regulate IL-27-induced type 1 regulatory T-cell differentiation. Proc Natl Acad Sci USA 110(19):7802–7807. https://doi.org/10.1073/pnas.1211776110
Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, Barsheshet Y, Karp CL, Karin N (2014) CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest 124(5):2009–2022. https://doi.org/10.1172/JCI71951
Butti E, Bergami A, Recchia A, Brambilla E, Del Carro U, Amadio S, Cattalini A, Esposito M, Stornaiuolo A, Comi G, Pluchino S, Mavilio F, Martino G, Furlan R (2008) IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther 15(7):504–515. https://doi.org/10.1038/gt.2008.10
Lian G, Gnanaprakasam JR, Wang T, Wu R, Chen X, Liu L, Shen Y, Yang M, Yang J, Chen Y, Vasiliou V, Cassel TA, Green DR, Liu Y, Fan TW, Wang R (2018) Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. Elife 7:e36158. https://doi.org/10.7554/eLife.36158
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186(6):3299–3303. https://doi.org/10.4049/jimmunol.1003613
Priyadharshini B, Loschi M, Newton RH, Zhang JW, Finn KK, Gerriets VA, Huynh A, Rathmell JC, Blazar BR, Turka LA (2018) Cutting edge: TGF-beta and phosphatidylinositol 3-kinase signals modulate distinct metabolism of regulatory T cell subsets. J Immunol 201(8):2215–2219. https://doi.org/10.4049/jimmunol.1800311
Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, Apovian CM, Snyder-Cappione J, Hawk GS, Fleeman RM, Pihl RMF, Thompson K, Belkina AC, Cui L, Proctor EA, Kern PA, Nikolajczyk BS (2020) Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab 32(1):44-55.e46. https://doi.org/10.1016/j.cmet.2020.04.015
Zhang D, Jin W, Wu R, Li J, Park SA, Tu E, Zanvit P, Xu J, Liu O, Cain A, Chen W (2019) High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-beta cytokine activation. Immunity 51(4):671-681.e675. https://doi.org/10.1016/j.immuni.2019.08.001
Alissafi T, Kalafati L, Lazari M, Filia A, Kloukina I, Manifava M, Lim JH, Alexaki VI, Ktistakis NT, Doskas T, Garinis GA, Chavakis T, Boumpas DT, Verginis P (2020) Mitochondrial oxidative damage underlies regulatory T cell defects in autoimmunity. Cell Metab 32(4):591-604.e597. https://doi.org/10.1016/j.cmet.2020.07.001
Al-Nashmi M, Taha S, Salem AH, Alsharoqi I, Bakhiet M (2018) Distinct HLA class I and II genotypes and haplotypes are associated with multiple sclerosis in Bahrain. Biomed Rep 9(6):531–539. https://doi.org/10.3892/br.2018.1164
Enz LS, Zeis T, Schmid D, Geier F, van der Meer F, Steiner G, Certa U, Binder TMC, Stadelmann C, Martin R, Schaeren-Wiemers N (2020) Increased HLA-DR expression and cortical demyelination in MS links with HLA-DR15. Neurol Neuroimmunol Neuroinflamm 7(2):e656. https://doi.org/10.1212/NXI.0000000000000656
Sturner KH, Siembab I, Schon G, Stellmann JP, Heidari N, Fehse B, Heesen C, Eiermann TH, Martin R, Binder TM (2019) Is multiple sclerosis progression associated with the HLA-DR15 haplotype? Mult Scler J Exp Transl Clin 5(4):2055217319894615. https://doi.org/10.1177/2055217319894615
Louie KA, Weiner LP, Du J, Kochounian HH, Fling SP, Wei W, McMillan M (2005) Cell-based gene therapy experiments in murine experimental autoimmune encephalomyelitis. Gene Ther 12(14):1145–1153. https://doi.org/10.1038/sj.gt.3302503
Keeler GD, Kumar S, Palaschak B, Silverberg EL, Markusic DM, Jones NT, Hoffman BE (2018) Gene therapy-induced antigen-specific Tregs inhibit neuro-inflammation and reverse disease in a mouse model of multiple sclerosis. Mol Ther 26(1):173–183. https://doi.org/10.1016/j.ymthe.2017.09.001
Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, Watkins LR, Leinwand L, Milligan ED, Van Dam AM (2009) Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental multiple sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun 23(1):92–100. https://doi.org/10.1016/j.bbi.2008.09.004
Garren H, Robinson WH, Krasulova E, Havrdova E, Nadj C, Selmaj K, Losy J, Nadj I, Radue EW, Kidd BA, Gianettoni J, Tersini K, Utz PJ, Valone F, Steinman L, Group BHTS (2008) Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann Neurol 63(5):611–620. https://doi.org/10.1002/ana.21370
Talla V, Koilkonda R, Guy J (2020) Gene therapy with single-subunit yeast NADH-ubiquinone oxidoreductase (NDI1) improves the visual function in experimental autoimmune encephalomyelitis (EAE) mice model of multiple sclerosis (MS). Mol Neurobiol 57(4):1952–1965. https://doi.org/10.1007/s12035-019-01857-6
Moghadam S, Erfanmanesh M, Esmaeilzadeh A (2017) Interleukin 35 and hepatocyte growth factor; as a novel combined immune gene therapy for multiple sclerosis disease. Med Hypotheses 109:102–105. https://doi.org/10.1016/j.mehy.2017.09.017
Hamana A, Takahashi Y, Tanioka A, Nishikawa M, Takakura Y (2018) Safe and effective interferon-beta gene therapy for the treatment of multiple sclerosis by regulating biological activity through the design of interferon-beta-galectin-9 fusion proteins. Int J Pharm 536(1):310–317. https://doi.org/10.1016/j.ijpharm.2017.12.010
Islam MA, Kundu S, Hassan R (2020) Gene therapy approaches in an autoimmune demyelinating disease: multiple sclerosis. Curr Gene Ther 19(6):376–385. https://doi.org/10.2174/1566523220666200306092556
Zhu J, Liu JQ, Liu Z, Wu L, Shi M, Zhang J, Davis JP, Bai XF (2018) Interleukin-27 gene therapy prevents the development of autoimmune encephalomyelitis but fails to attenuate established inflammation due to the expansion of CD11b(+)Gr-1(+) myeloid cells. Front Immunol 9:873. https://doi.org/10.3389/fimmu.2018.00873
Noori-Zadeh A, Mesbah-Namin SA, Saboor-Yaraghi AA (2017) Epigenetic and gene expression alterations of FOXP3 in the T cells of EAE mouse model of multiple sclerosis. J Neurol Sci 375:203–208. https://doi.org/10.1016/j.jns.2017.01.060
Aslani S, Jafari N, Javan MR, Karami J, Ahmadi M, Jafarnejad M (2017) Epigenetic modifications and therapy in multiple sclerosis. Neuromol Med 19(1):11–23. https://doi.org/10.1007/s12017-016-8422-x
Gholamzad M, Ebtekar M, Ardestani MS, Azimi M, Mahmodi Z, Mousavi MJ, Aslani S (2019) A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future. Inflamm Res 68(1):25–38. https://doi.org/10.1007/s00011-018-1185-0
O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D (2010) MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33(4):607–619. https://doi.org/10.1016/j.immuni.2010.09.009
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G (2009) MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 10(12):1252–1259. https://doi.org/10.1038/ni.1798
Potenza N, Mosca N, Mondola P, Damiano S, Russo A, De Felice B (2018) Human miR-26a-5p regulates the glutamate transporter SLC1A1 (EAAT3) expression. Relevance in multiple sclerosis. Biochim Biophys Acta 1864(1):317–323. https://doi.org/10.1016/j.bbadis.2017.09.024
Thamilarasan M, Koczan D, Hecker M, Paap B, Zettl UK (2012) MicroRNAs in multiple sclerosis and experimental autoimmune encephalomyelitis. Autoimmun Rev 11(3):174–179. https://doi.org/10.1016/j.autrev.2011.05.009
Acknowledgements
This work was supported by Natural Science Foundation of Hunan Province Grant (Grant No. 2019JJ40243), Key Laboratory of Ecological Environment and Critical Human Diseases Prevention of Hunan Province Department of Education (20K107).
Author information
Authors and Affiliations
Contributions
We have added a co-author to the revised manuscript. His name is GW. In the process of revising the manuscript, he provided us with the drug company and FDA approval date. Based on the above contribution to the revision of the manuscript, we newly added him as a co-author.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Lian, G., Wang, G. et al. A review of possible therapies for multiple sclerosis. Mol Cell Biochem 476, 3261–3270 (2021). https://doi.org/10.1007/s11010-021-04119-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04119-z