Abstract
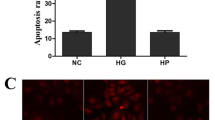
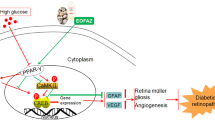
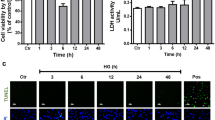
NADPH oxidase (NOX) is a main producers of reactive oxygen species (ROS) that may contribute to the early pathogenesis of diabetic retinopathy (DR). ROS has harmful effects on endogenous neuro-survival factors brain-derived neurotrophic factor (BDNF) and sirtuin 1 (SIRT1) are necessary for the growth and survival of the retina. The role of NOX isoforms NOX4 in triggering ROS in DR is not clear. Here we determine the protective effects of a plant-derived NOX inhibitor apocynin (APO) on NOX4-induced ROS production which may contribute to the depletion of survival factors BDNF/SIRT1 or cell death in the diabetic retinas. Human retinal Müller glial cells (MGCs) were treated with hypoxia mimetic agent cobalt chloride (CoCl2) in the absence or presence of APO. Molecular analysis demonstrates that NOX4 is upregulated in CoCl2-treated MGCs and in the diabetic retinas. Increased NOX4 was accompanied by the downregulation of BDNF/SIRT1 expression or in the activation of apoptotic marker caspase-3. Whereas, APO treatment downregulates NOX4 and subsequently upregulates BDNF/SIRT1 or alleviate caspase-3 expression. Accordingly, in the diabetic retina we found a positive correlation in NOX4 vs ROS (p = 0.025; R2 = 0.488) and caspase-3 vs ROS (p = 0.04; R2 = 0.428); whereas a negative correlation in BDNF vs ROS (p = 0.009; R2 = 0.596) and SIRT1 vs ROS (p = 0.0003; R2 = 0.817) respectively. Taken together, NOX4-derived ROS could be a main contributor in downregulating BDNF/SIRT1 expression or in the activation of caspase-3. Whereas, APO treatment may minimize the deleterious effects occurring due to hyperglycemia and/or diabetic mimic hypoxic condition in early pathogenesis of DR.






Similar content being viewed by others
References
van der Heijden AA et al (2020) Prediction models for development of retinopathy in people with type 2 diabetes: systematic review and external validation in a Dutch primary care setting. Diabetologia
Santiago AR et al (2018) Sweet stress: coping with vascular dysfunction in diabetic retinopathy. Front Physiol 9:820
Nawaz IM et al (2019) Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Prog Retin Eye Res
Qu ZA et al (2020) SIRT2 inhibits oxidative stress and inflammatory response in diabetic osteoarthritis. Eur Rev Med Pharmacol Sci 24(6):2855–2864
Ola MS et al (2013) Neurodegeneration and neuroprotection in diabetic retinopathy. Int J Mol Sci 14(2):2559–2572
Fresta CG et al (2020) A new human blood-retinal barrier model based on endothelial cells, pericytes, and astrocytes. Int J Mol Sci 21(5)
Yang Q et al (2020) HDAC6 inhibitor Cay10603 inhibits high glucose-induced oxidative stress, inflammation and apoptosis in retinal pigment epithelial cells via regulating NF-kappaB and NLRP3 inflammasome pathway. Gen Physiol Biophys 39(2):169–177
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5(5):415–418
Peng JJ et al (2019) Diabetic retinopathy: focus on NADPH oxidase and its potential as therapeutic target. Eur J Pharmacol 853:381–387
Li J et al (2010) Inhibition of reactive oxygen species by lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes 59(6):1528–1538
Jiao W et al (2019) Activation of the NotchNox4reactive oxygen species signaling pathway induces cell death in high glucosetreated human retinal endothelial cells. Mol Med Rep 19(1):667–677
Ding L et al (2014) Peroxisome proliferator-activated receptor α protects capillary pericytes in the retina. Am J Pathol 184(10):2709–2720
Liu Q et al (2019) Salutary effect of fenofibrate on type 1 diabetic retinopathy via inhibiting oxidative stress-mediated Wnt/β-catenin pathway activation. Cell Tissue Res 376(2):165–177
Deliyanti D et al (2020) Nox (NADPH oxidase) 1, Nox4, and Nox5 promote vascular permeability and neovascularization in retinopathy. Hypertension 75(4):1091–1101
Shalaby L et al (2020) Role of endothelial ADAM17 in early vascular changes associated with diabetic retinopathy. J Clin Med 9(2)
Li J, Wang JJ, Zhang SX (2015) NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. J Diabetes Res 2015:963289
Meng W et al (2018) A genome-wide association study suggests new evidence for an association of the NADPH oxidase 4 (NOX4) gene with severe diabetic retinopathy in type 2 diabetes. Acta Ophthalmol 96(7):e811–e819
Cohen-Cory S et al (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70(5):271–288
Afarid M, Namvar E, Sanie-Jahromi F (2020) Diabetic retinopathy and BDNF: a review on its molecular basis and clinical applications. J Ophthalmol 2020:1602739
Seki M et al (2005) Müller cells as a source of brain-derived neurotrophic factor in the retina: noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Müller cells. Neurochem Res 30(9):1163–1170
Kashiwagi F et al (2000) Effects of brain-derived neurotrophic factor and neurotrophin-4 on isolated cultured retinal ganglion cells: evaluation by flow cytometry. Invest Ophthalmol Vis Sci 41(8):2373–2377
Zhang CW et al (2005) CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci 46(4):1497–1503
Skytt DM et al (2016) Glia-neuron interactions in the retina can be studied in Cocultures of Müller cells and retinal ganglion cells. Biomed Res Int 2016:1087647
Suzumura A et al (2020) N-3 fatty acid and its metabolite 18-HEPE ameliorate retinal neuronal cell dysfunction by enhancing Muller BDNF in diabetic retinopathy. Diabetes 69(4):724–735
Sasaki M et al (2010) Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 53(5):971–979
Mansour-Robaey S et al (1994) Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A 91(5):1632–1636
Salminen A, Kaarniranta K, Kauppinen A (2013) Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci 14(2):3834–3859
Mohammad G et al (2019) Cross-talk between Sirtuin 1 and the Proinflammatory mediator high-mobility group Box-1 in the regulation of blood-retinal barrier breakdown in diabetic retinopathy. Curr Eye Res 44(10):1133–1143
Mishra M, Duraisamy AJ, Kowluru RA (2018) Sirt1: a Guardian of the development of diabetic retinopathy. Diabetes 67(4):745–754
Nimmagadda VK et al (2013) Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J Immunol 190(9):4595–4607
Tang X et al (2020) Resveratrol mitigates sevoflurane-induced neurotoxicity by the SIRT1-dependent regulation of BDNF expression in developing mice. Oxidative Med Cell Longev 2020:9018624
Cao Y et al (2017) SIRT1 regulates cognitive performance and ability of learning and memory in diabetic and nondiabetic models. J Diabetes Res 2017:7121827
Mohr S et al (2002) Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes 51(4):1172–1179
Carmody RJ, Cotter TG (2000) Oxidative stress induces caspase-independent retinal apoptosis in vitro. Cell Death Differ 7(3):282–291
Li GY, Fan B, Su GF (2009) Acute energy reduction induces caspase-dependent apoptosis and activates p53 in retinal ganglion cells (RGC-5). Exp Eye Res 89(4):581–589
Yuan D et al (2014) Edaravone protect against retinal damage in streptozotocin-induced diabetic mice. PLoS One 9(6):e99219
Ola MS et al (2013) Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell Mol Neurobiol 33(3):359–367
Mohammad G, Siddiquei MM, Abu El-Asrar AM (2013) Poly (ADP-ribose) polymerase mediates diabetes-induced retinal neuropathy. Mediat Inflamm 2013:510451
Chignalia AZ, Weinberg G, Dull RO (2020) Norepinephrine induces lung microvascular endothelial cell death by NADPH oxidase-dependent activation of Caspase-3. Oxidative Med Cell Longev 2020:2563764
Abu El-Asrar AM et al (2013) Expression of lysophosphatidic acid, autotaxin and acylglycerol kinase as biomarkers in diabetic retinopathy. Acta Diabetol 50(3):363–371
Kowluru RA, Mishra M (2015) Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta 1852(11):2474–2483
Ola MS et al (2012) Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complicat 26(1):56–64
Behl T, Kaur I, Kotwani A (2016) Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol 61(2):187–196
Gorin Y, Block K (2013) Nox as a target for diabetic complications. Clin Sci (Lond) 125(8):361–382
Mohammad G et al (2015) Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem 71(3):359–372
Mohammad G et al (2018) The poly(ADP-ribose)Polymerase-1 inhibitor 1,5-Isoquinolinediol attenuate diabetes-induced NADPH oxidase-derived oxidative stress in retina. J Ocul Pharmacol Ther 34(7):512–520
Jiao W et al (2019) Activation of the notch-Nox4-reactive oxygen species signaling pathway induces cell death in high glucose-treated human retinal endothelial cells. Mol Med Rep 19(1):667–677
Qiao Y, Fan CL, Tang MK (2017) Astragaloside IV protects rat retinal capillary endothelial cells against high glucose-induced oxidative injury. Drug Des Devel Ther 11:3567–3577
Van den Worm E et al (2001) Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur J Pharmacol 433(2–3):225–230
Ma MW et al (2018) NADPH oxidases in traumatic brain injury – promising therapeutic targets? Redox Biol 16:285–293
Qin YY et al (2018) Corrigendum to “Combined NADPH and the NOX inhibitor apocynin provides greater anti-inflammatory and neuroprotective effects in a mouse model of stroke” [Free Radic Biol Med 104 (2017) 333-345]. Free Radic Biol Med 115:498–499
Jin HZ et al (2019) Apocynin alleviates lung injury by suppressing NLRP3 inflammasome activation and NF-kappaB signaling in acute pancreatitis. Int Immunopharmacol 75:105821
Tan YC et al (2020) Apocynin and catalase prevent hypertension and kidney injury in cyclosporine A-induced nephrotoxicity in rats. PLoS One 15(4):e0231472
Liao CR et al (2020) Advanced oxidation protein products increase TNF-alpha and IL-1beta expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol 28:101306
Yang X et al (2020) Apocynin attenuates acute kidney injury and inflammation in rats with acute Hypertriglyceridemic pancreatitis. Dig Dis Sci 65(6):1735–1747
Kurosawa T et al (2020) Evaluation of apocynin in vitro on high glucose-induced oxidative stress on tenocytes. Bone Joint Res 9(1):23–28
Sun J et al (2015) Apocynin suppression of NADPH oxidase reverses the aging process in mesenchymal stem cells to promote osteogenesis and increase bone mass. Sci Rep 5:18572
Olukman M et al (2018) Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats. Neural Regen Res 13(9):1657–1664
Olukman M et al (2010) Apocynin restores endothelial dysfunction in streptozotocin diabetic rats through regulation of nitric oxide synthase and NADPH oxidase expressions. J Diabetes Complicat 24(6):415–423
Urner S et al (2020) NADPH oxidase inhibition: preclinical and clinical studies in diabetic complications. Antioxid Redox Signal 33(6):415–434
Wu M et al (2012) Oxidative and endoplasmic reticulum stresses mediate apoptosis induced by modified LDL in human retinal Muller cells. Invest Ophthalmol Vis Sci 53(8):4595–4604
Chen H et al (2018) Myeloid differentiation protein 2 induced retinal ischemia reperfusion injury via upregulation of ROS through a TLR4-NOX4 pathway. Toxicol Lett 282:109–120
Seki M et al (2004) Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes 53(9):2412–2419
Abu El-Asrar AM et al (2013) High-mobility group box-1 induces decreased brain-derived neurotrophic factor-mediated neuroprotection in the diabetic retina. Mediat Inflamm 2013
Wang S et al (2017) SIRT1 activation inhibits hyperglycemia-induced apoptosis by reducing oxidative stress and mitochondrial dysfunction in human endothelial cells. Mol Med Rep 16(3):3331–3338
Gao J et al (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466(7310):1105–1109
Wang P, Xia F (2015) EPO protects Müller cell under high glucose state through BDNF/TrkB pathway. Int J Clin Exp Pathol 8(7):8083–8090
Ma Y, Li W, Yin Y (2015) AST IV inhibits H2O2-induced human umbilical vein endothelial cell apoptosis by suppressing Nox4 expression through the TGF-β1/Smad2 pathway. Int J Mol Med 35(6):1667–1674
Su J et al (2018) Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats. Sci Rep 8(1):12620
Kern TS, Barber AJ (2008) Retinal ganglion cells in diabetes. J Physiol 586(18):4401–4408
Wang Y et al (2019) Apocynin ameliorates diabetic retinopathy in rats: involvement of TLR4/NF-κB signaling pathway. Int Immunopharmacol 73:49–56
Cecilia OM et al (2019) Oxidative stress as the main target in diabetic retinopathy pathophysiology. J Diabetes Res 2019:8562408
Kowluru RA, Chan PS (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007:43603
Simonyi A et al (2012) The neuroprotective effects of apocynin. Front Biosci (Elite Ed) 4:2183–2193
Liu F et al (2020) In vivo and in silico characterization of apocynin in reducing organ oxidative stress: a pharmacokinetic and pharmacodynamic study. Pharmacol Res Perspect 8(4):e00635
Acknowledgement
The authors thank for funding by Dr. Nasser Al-Rasheed Research Chair in Ophthalmology (Abu El-Asrar A.M.), King Saud University, KSA.
Funding
Sources of Support: This work was supported by Dr. Nasser Al-Rasheed Research Chair in Ophthalmology (Abu El-Asrar A.M.), King Saud University, K.S.A.
Author information
Authors and Affiliations
Contributions
AJ and MIN contributed to the conception, design of the study, and drafted the manuscript. MMS, AJ, and MIN performed the experiments and conducted the data analysis. AMAE edited the manuscript and provided comments. AJ is responsible for the integrity and accuracy of the work, as well have full access to all the data in the study. All authors read and revised the manuscript for intellectual content and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of the interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, A., Nawaz, M.I., Siddiquei, M.M. et al. Apocynin ameliorates NADPH oxidase 4 (NOX4) induced oxidative damage in the hypoxic human retinal Müller cells and diabetic rat retina. Mol Cell Biochem 476, 2099–2109 (2021). https://doi.org/10.1007/s11010-021-04071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04071-y