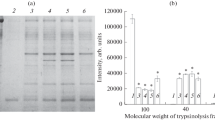
Abstract
The enzyme betaine aldehyde dehydrogenase (BADH EC 1.2.1.8) catalyzes the synthesis of glycine betaine (GB), an osmolyte and osmoprotectant. Also, it participates in several metabolic pathways in humans. All BADHs known have cysteine in the active site involved in the aldehyde binding, whereas the porcine kidney enzyme (pkBADH) also has a neighborhood cysteine, both sensitive to oxidation. The antineoplastic and immuno-suppressant pre-drug cyclophosphamide (CTX), and its bioactivation products, have two highly oxidating chlorine atoms. This work aimed to analyze the effect of CTX in the activity of porcine kidney betaine aldehyde dehydrogenase. PkBADH was incubated with varying CTX concentration (0 to 2.0 mM) at 25 °C and lost 50 % of its activity with 2.0 mM CTX. The presence of the coenzyme NAD+ (0.5 mM) decreased 95% the activity in 2.0 mM CTX. The substrate betaine aldehyde (0.05 and 0.4 mM, and the products NADH (0.1–0.5 mM) and GB (1 and 10 mM) did not have an effect on the enzyme inactivation by CTX. The reducing agents, dithiothreitol and β-mercaptoethanol, reverted the pkBADH inactivation, but reduced glutathione (GSH) was unable to restore the enzyme activity. Molecular docking showed that CTX could enter at the enzyme active site, where its chlorine atoms may interact with the catalytic and the neighboring cysteines. The results obtained show that CTX inactivates the pkBADH due to oxidation of the catalytic cysteine or because it oxidizes catalytic and neighborhood cysteine, forming a disulfide bridge with a concomitant decrease in the activity of the enzyme.






Similar content being viewed by others
References
Kurys G, Ambroziak W, Pietruszko R (1989) Human aldehyde dehydrogenase. Purification and characterization of a third with low Km for γ-aminobutyraldehyde. J Biol Chem 264(8):4715–4721
Chern MK, Pietruszko R (1995) Human aldehyde dehydrogenase E3 isozyme is a betaine aldehyde dehydrogenase. Biochem Biophys Res Commun 213:561–8
Vasiliou V, Nebert DW (2005) Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genom 2:138–143
Lin S, Chen J (1996) Human gamma-aminobutyraldehyde dehydrogenase (ALDH9): cDNA sequence, genomic organization, polymorphism, chromosomal localization, and tissue expression. Genomics 34:376–380
Izaguirre G, Kikonyogo A, Pietruszko R (1997) Tissue distribution of human aldehyde dehydrogenase E3 (ALDH9): comparison of enzyme activity with E3 protein and mRNA distribution. Comp Biochem Physiol 118B(1):59–64
Chern M, Pietruszko R (1999) Evidence for mitochondrial localization of betaine aldehyde dehydrogenase in rat liver: purification, characterization, and comparison with human cytoplasmic E3 isozyme. Biochem Cell Biol 77(3):179–87
Guzman-Partida AM, Valenzuela-Soto EM (1998) Porcine kidney betaine aldehyde dehydrogenase: purification and properties. Comp Biochem Physiol Part B 119:485–491
Neuhofer W, Beck FX (2006) Survival in hostile environment: strategies of renal medullary cells. Physiology 21:171–180
Burg MB, Ferraris JD (2008) Intracellular organic osmolytes: function and regulation. J Biol Chem 283:7309–7313
Hosseiniyan Khatibi SM, Zununi Vahed F, Sharifi S, Ardalan M, Mohajel Shoja M, Zununi Vahed S (2019) Osmolytes resist against harsh osmolarity: something old something new. Biochimie 158:156–164
Figueroa-Soto C, Valenzuela-Soto EM (2018) Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in celular metabolism. Biochimie. 147:89–97
Kültz D (2004) Hyperosmolality triggers oxidative damage in kidney cells. Proc Natl Acad Sci USA 101:9177–9179
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C et al (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34:336–344. https://doi.org/10.1002/eji.200324181
Emadi A, Jones RJ, Brodsky RA (2009) Cyclophosphamide and cancer: golden anniversary. Natr Rev Clin Oncol 6(11):638–47
Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L (2011) Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol 33:369–83
Brummaier T, Pohanka E, Studnicka-Benke A, Pieringer H (2013) Using cyclophosphamide in inflammatory rheumatic diseases. Eur J Intern Med 24(7):590–596
Gupta R, Cristea M, Frankel P, Ruel C, Chen C, Wang Y et al (2019) Randomized trial of oral cyclophosphamide versus oral cyclophosphamide with celecoxib for recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer. Cancer Treat Res Commun 2:100155. https://doi.org/10.1016/j.ctarc.2019.100155
Rodriguez-Antona C, Ingelman-Sundberg M (2006) Cytochrome P450 pharmacogenetics and cancer. Oncogene 25:1679–1691. https://doi.org/10.1038/sj.onc.1209377
El-Serafi I, Afsharian P, Moshfegh A, Hassan M, Terelius Y (2015) Cytochrome P450 oxidoreductase influences cyp2b6 activity in cyclophosphamide bioactivation. PLoS One 10(11):e0141979. https://doi.org/10.1371/journal.pone.0141979
Helsby NA, Yong M, van Kan M, de Zoysa JR, Burns KE (2019) The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br J Clin Pharmacol 85:1925–1934. https://doi.org/10.1111/bcp.14031
Song J, Liu L, Li L, Liu J, Song Y (2013) Protective effects of lipoic acid and mesna on cyclophosphamide- induced haemorrhagic cystitis in mice. Cell Biochem Funct 32:125–132
Caglayan C (2019) The effects of naringin on different cyclophosphamide-induced organ toxicities in rats: investigation of changes in some metabolic enzyme activities. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-05915-3
Omdal R, Husby G, Koldingsnes W (1993) Intracenous and oral cyclophosmamide pulse therapy in rheumatic deseases: side effect and complications. Clin Exp Rheumatol 11:281–8
Bonella B, Warley F, Gutierrez P, Ferreyro B (2017) Hyponatremia induced by high dose cyclophosphamide therapy: a retrospective cohort study Cyclophosphamide and hyponatremia. Rev Fac Cien Med 74:201–206
Morandi P, Ruffini PA, Benvenuto GM, Raimondi R, Fosser V (2005) Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant 35:323–334
Motoki N, Shimizu T, Akazawa Y, Saito S, Tanaka M, Yanagisawa R et al (2010) Increased pretransplant QT dispersion as a risk factor for the development of cardiac complications during and after preparative conditioning for pediatric allogeneic hematopoietic stem cell transplantation. Pediatr Transplant 14:986–992
Zhai X, Zhang Z, Liu W, Liu B, Zhang R, Wang W et al (2018) Protective effect of ALDH2 against cyclophosphamide-induced acute hepatotoxicity via attenuating oxidative stress and reactive aldehydes. Biochem Biophys Res Commun 499(1):93–98
Muñoz-Bacasehua C, Rosas-Rodríguez JA, Arvizu-Flores AA, Stephens-Camacho A, Soñanez-Organis JG, Figueroa-Soto CG, Valenzuela-Soto EM (2020) Heterogeneity of active sites in porcine kidney betaine aldehyde dehydrogenase is modulated by potassium. J Molec Recongnit. https://doi.org/10.1002/jmr.2869
Laemmli UK (1970) Cleaveage of Estructural protein during the assembly of the head of bacteriophage T4. Nature 227:680–685
Muñoz-Bacasehua C, Rosas-Rodríguez JA, Arvizu-Flores AA, Valenzuela-Soto EM (2020) Role of potassium levels in pkBADH heterogeneity of NAD+ binding site. J Bioenerg Biomembr 52(2):61–70
Velasco-García R, Zaldívar-Machorro VJ, Mújica-Jiménez C, GonzálezSegura L, Muñoz-Clares RA (2006) Disulfiram irreversibly aggregates betaine aldehyde dehydrogenase a potential target for antimicrobial agents against Pseudomonas aeruginosa. Biochem Biophys Res Commun 341:408e415
Valenzuela-Soto EM, Velasco-García R, Mújica-Jiménez C, Muñoz-Clares RA (2003) Monovalent cations requirements for the stability of betaine aldehyde dehydrogenase from Pseudomonas aeruginosa, porcine kidney and amaranth leaves. Chem-Biol Interact 143:139–148
Rosas-Rodríguez JA, Valenzuela-Soto EM (2011) Inactivation of porcine kidney betaine aldehyde dehydrogenase by hydrogen peroxide. Chem-Biol Interact 191(1–3):159–64
Ayala-Castro H, Valenzuela-Soto EM, Figueroa-Soto C, Muñoz-Clares R (2007) Complex, unusual conformational changes in kidney betaine aldehyde dehydrogenase suggested by chemical modification with disulfiram. Arch Biochem Biophys 468(2):167–73
Figueroa-Soto C, Valenzuela-Soto EM (2000) Kinetic study of porcine kidney betaine aldehyde dehydrogenase. Biochem Biophys Res Commun 269(2):596–603
Muñoz-Clares RA, González-Segura L, Mújica-Jiménez C, Contreras-Dı́az L (2003) Ligand-induced conformational changes of betaine aldehyde dehydrogenase from Pseudomonas aeruginosa and Amaranthus hypochondriacus L. leaves affecting the reactivity of the catalytic thiol. Chem-Biol Interact 143–144:129–137
Modal R, Husky G, Koldingsnes W (1993) Intravenous and oral cyclophosphamide pulse therapy in rheumatic diseases: side effects and complications. Clin Exp Rheumatol 11(8):283–290
Figueroa-Soto CG, Lopez-Cervantes G, Valenzuela-Soto EM (1999) Immunolocalization of betaine aldehyde dehydrogenase in porcine kidney. Biochem Biophys Res Commun 258:732–736
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–16
Behrens KA, Jania LA, Snouwaert JN, Nguyen M, Moy SS, Tikunov AP, Macdonald JM, Koller BH (2019) Beyond detoxification: Pleiotropic functions of multiple glutathione S-transferase isoforms protect mice against a toxic electrophile. PLoS One 14(11):e0225449. https://doi.org/10.1371/journal.pone.0225449
Acknowledgements
R. Cruz-Valencia gratefully acknowledges a scholarship from CONACyT for graduate studies.
Funding
Authors did not receive funding to this work.
Author information
Authors and Affiliations
Contributions
RCV performed the experiments, EMVS, JARR, AAAF conceived the experiments, RCV, EMVS, JARR, AAAF analyzed the data, RCV and EMVS wrote the original draft of manuscript, all authors revised and edited the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cruz-Valencia, R., Arvizu-Flores, A.A., Rosas-Rodríguez, J.A. et al. Effect of the drug cyclophosphamide on the activity of porcine kidney betaine aldehyde dehydrogenase. Mol Cell Biochem 476, 1467–1475 (2021). https://doi.org/10.1007/s11010-020-04010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-04010-3