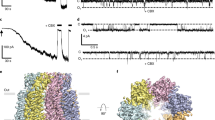
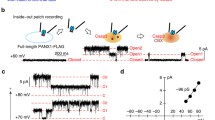
Abstract
Pannexins, large non-gap junction super family exists in vertebrates, play multiple roles in different cellular functions through their ATP release. Panx1-mediated adenosine 5′-triphosphate (ATP) release plays a vital role in physiological and pathophysiological conditions and is known major extracellular molecule in purinergic signaling. To modulate their function in vivo, a proper regulation of channel is necessary. Post-translational modifications are considered to be some regulating mechanisms for PANX1, while PANX2, PANX3 have been uncharacterized to date. Through their significant evidences, PANXs exclude from gap junction and conduits ATP release and other cellular molecules from cells by various mechanisms. PANX1 is most extensive characterized and implicated in ATP signaling and inflammatory processes. Despite the constant advances, much significance of PANX1 in physiological processes remains elusive. Recently, various research groups along with our group have reported the Cryo-EM structure of Panx1 channel and uncovered the hidden functions in structure–function mechanism as well as to provide the clear understanding in physiological and pathophysiological roles. These research groups reported the novel heptameric structure with contains 4 transmembrane helices (TM), two extracellular loops and one intracellular loop with N and C terminus located at the intracellular side. In addition, the structure contains a large pore of which an inhibitor CBX act as a plug that blocking the passage of substrate. In this context, this review will present current mechanistic understanding in structure and function together with significant physiological roles particularly ATP release in health and disease. As such, this review emphasizes on recent functional properties associated with novel heptameric channel and demystifies channel-mediated ATP release function.





Similar content being viewed by others
References
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1 beta release by the ATP-gated P2X(7) receptor. EMBO J 25:5071–5082. https://doi.org/10.1038/sj.emboj.7601378
Scemes E, Suadicani SO, Dahl G, Spray DC (2007) Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3:199–208. https://doi.org/10.1017/s1740925x08000069
Alhouayek M, Sorti R, Gilthorpe JD, Fowler CJ (2019) Role of pannexin-1 in the cellular uptake, release and hydrolysis of anandamide by T84 colon cancer cells. Sci Rep 9:7622. https://doi.org/10.1038/s41598-019-44057-x
Velasquez S, Eugenin EA (2014) Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front Physiol 5:96. https://doi.org/10.3389/fphys.2014.00096
Panchin Y et al (2000) A ubiquitous family of putative gap junction molecules. Curr Biol 10:R473-474
Phelan P (2005a) Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta 1711:225–245. https://doi.org/10.1016/j.bbamem.2004.10.004
Barbe MT, Monyer H, Bruzzone R (2006) Cell-cell communication beyond connexins: the pannexin channels. Physiology 21:103–114. https://doi.org/10.1152/physiol.00048.2005
Panchin YV (2005a) Evolution of gap junction proteins—the pannexin alternative. J Exp Biol 208:1415–1419. https://doi.org/10.1242/jeb.01547
Dubyak GR (2009) Both sides now: multiple interactions of ATP with pannexin-1 hemichannels. Focus on A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol 296:C235-241. https://doi.org/10.1152/ajpcell.00639.2008
D’Hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B (2009) Pannexins, distant relatives of the connexin family with specific cellular functions? BioEssays 31:953–974. https://doi.org/10.1002/bies.200800236
Ma W et al (2012) Pannexin 1 forms an anion-selective channel. Pflugers Arch 463:585–592. https://doi.org/10.1007/s00424-012-1077-z
Bond SR, Naus CC (2014) The pannexins: past and present. Front Physiol 5:58. https://doi.org/10.3389/fphys.2014.00058
Riquelme MA et al (2013) The ATP required for potentiation of skeletal muscle contraction is released via pannexin hemichannels. Neuropharmacology 75:594–603. https://doi.org/10.1016/j.neuropharm.2013.03.022
Chiu YH, Schappe MS, Desai BN, Bayliss DA (2018) Revisiting multimodal activation and channel properties of Pannexin 1. J Gen Physiol 150(1):19–39. https://doi.org/10.1085/jgp.201711888
Iwamoto T et al (2010) Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem 285:18948–18958. https://doi.org/10.1074/jbc.M110.127027
Bhaskaracharya A et al (2014) Probenecid blocks human P2X7 receptor-induced dye uptake via a Pannexin-1 independent mechanism. PLoS ONE 9:e93058. https://doi.org/10.1371/journal.pone.0093058
Ma W, Hui H, Pelegrin P, Surprenant A (2009) Pharmacological characterization of Pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 328:409–418. https://doi.org/10.1124/jpet.108.146365
Bao L, Locovei S, Dahl G (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572:65–68. https://doi.org/10.1016/j.febslet.2004.07.009
Li S, Bjelobaba I, Stojilkovic SS (2018) Interactions of Pannexin1 channels with purinergic and NMDA receptor channels. Biochim Biophys Acta Biomembr 1860(1):166–173. https://doi.org/10.1016/j.bbamem.2017.03.025
Chekeni FB et al (2010) Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis. Nature 467:863-U136. https://doi.org/10.1038/nature09413
Seminario-Vidal L et al (2011) Rho signaling regulates Pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286:26277–26286. https://doi.org/10.1074/jbc.M111.260562
Thompson RJ et al (2008) Activation of Pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322:1555–1559. https://doi.org/10.1126/science.1165209
Lamb IR, Novielli NM, Murrant CL (2018) Pannexin’s role in mediating skeletal muscle active hyperaemia. Faseb J 32:703–705
Saez JC, Cisterna BA, Vargas A, Cardozo CP (2015) Regulation of pannexin and connexin channels and their functional role in skeletal muscles. Cell Mol Life Sci 72:2929–2935. https://doi.org/10.1007/s00018-015-1968-1
Dando R, Roper SD (2009) Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol 587:5899–5906. https://doi.org/10.1113/jphysiol.2009.180083
Marginedas-Freixa I et al (2018) Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. Sci Rep 8:1–13. https://doi.org/10.1038/s41598-018-29885-7
Penuela S, Celetti SJ, Bhalla R, Shao Q, Laird DW (2008) Diverse subcellular distribution profiles of pannexin1 and pannexin3. Cell Commun Adhes 15:133–142. https://doi.org/10.1080/15419060802014115
Penuela S, Bhalla R, Nag K, Laird DW (2009) Glycosylation regulates Pannexin intermixing and cellular localization. Mol Biol Cell 20:4313–4323. https://doi.org/10.1091/mbc.E09-01-0067
Pelegrin P, Surprenant A (2009) The P2X(7) receptor-Pannexin connection to dye uptake and IL-1beta release. Purinergic Signal 5(2):129–137. https://doi.org/10.1007/s11302-009-9141-7
Kanneganti TD, LamkanfiM KYG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26:433–443
Phelan P (2005b) Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta 1711(2):225–245
Panchin YV (2005b) Evolution of gap junction proteins–the pannexin alternative. J Exp Biol 208(Pt 8):1415–1419
Ma Z, Siebert AP, Cheung KH et al (2012) Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci USA 109(28):E1963–E1971. https://doi.org/10.1073/pnas.1204023109
Wang J, Dahl G (2010) SCAM analysis of Panx1 suggests a peculiar pore structure. J Gen Physiol 136(5):515–527
Yeager M, Gilula NB (1992) Membrane topology and quaternary structure of cardiac gap junction ion channels. J Mol Biol 223(4):929–948
Milks LC et al (1988) Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J 7(10):2967–2975
Kawate T, Gouaux E (2006) Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14:673–681
Michalski K, Syrjanen JL, Henze E, Kumpf J, Furukawa H, Kawate T (2020) The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. Elife 9:e54670. https://doi.org/10.7554/eLife.54670
Qu R, Dong L, Zhang J et al (2020) Cryo-EM structure of human heptameric Pannexin 1 channel. Cell Res. https://doi.org/10.1038/s41422-020-0298-5
Deng Z, He Z, Maksaev G, Bitter RM, Rau M, Fitzpatrick JAJ, Yuan P (2020) Cryo-EM structures of the ATP release channel pannexin 1. Nat Struct Mol Biol 27(4):373–381. https://doi.org/10.1038/s41594-020-0401-0
Jin Q, Zhang B, Zheng X et al (2020) Cryo-EM structures of human pannexin 1 channel. Cell Res. https://doi.org/10.1038/s41422-020-0310-0
Mou L, Ke M, Song M et al (2020) Structural basis for gating mechanism of Pannexin 1 channel. Cell Res 30(5):452–454. https://doi.org/10.1038/s41422-020-0313-x
Dahl G (2015) ATP release through pannexon channels. Philos Trans R Soc Lond B 370(1672):20140191. https://doi.org/10.1098/rstb.2014.0191
Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE (2014) Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev 66(2):513–569. https://doi.org/10.1124/pr.112.007351
Adamson SE, Leitinger N (2014) The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett 588(8):1416–1422. https://doi.org/10.1016/j.febslet.2014.03.009
Boyce AKJ, Epp AL, Nagarajan A (1860) LA Swayne (2018) Transcriptional and post-translational regulation of pannexins. Biochimica et Biophysica Acta (BBA) 1:72–82
Sandilos JK, Bayliss DA (2012) Physiological mechanisms for the modulation of Pannexin 1 channel activity. J Physiol 590(24):6257–6266. https://doi.org/10.1113/jphysiol.2012.240911
Adamson SE, Meher AK, Chiu YH, Sandilos JK, Oberholtzer NP, Walker NN, Hargett SR, Seaman SA, Peirce-Cottler SM, Isakson BE, McNamara CA, Keller SR, Harris TE, Bayliss DA, Leitinger N (2015) Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes. Mol Metab 4(9):610–618. https://doi.org/10.1016/j.molmet.2015.06.009
Ardiles AO, Flores-Muñoz C, Toro-Ayala G, Cárdenas AM, Palacios AG, Muñoz P, Fuenzalida M, Sáez JC, Martínez AD (2014) Pannexin 1 regulates bidirectional hippocampal synaptic plasticity in adult mice. Front Cell Neurosci 8:326. https://doi.org/10.3389/fncel.2014.00326
Prochnow N, Abdulazim A, Kurtenbach S, Wildförster V, Dvoriantchikova G, Hanske J et al (2012) Pannexin1 stabilizes synaptic plasticity and is needed for learning. PLoS ONE 7:e51767. https://doi.org/10.1371/journal.pone.0051767
Whyte-Fagundes P, Zoidl G (2018) Mechanisms of pannexin1 channel gating and regulation. Biochimica et Biophysica Acta (BBA) 1860(1):65–71
Ardiles AO, Flores-Muñoz C, Toro-Ayala G et al (2014) Pannexin 1 regulates bidirectional hippocampal synaptic plasticity in adult mice. Front Cell Neurosci 8:326. https://doi.org/10.3389/fncel.2014.00326
Basova LV, Tang X, Umasume T et al (2017) Manipulation of Panx1 activity increases the engraftment of transplanted lacrimal gland epithelial progenitor cells. Invest Ophthalmol Vis Sci 58(13):5654–5665. https://doi.org/10.1167/iovs.17-22071
Scemes E, Velíšek L, Velíšková J (2019) Astrocyte and neuronal pannexin1 contribute distinctly to seizures. ASN Neuro 11:1759091419833502. https://doi.org/10.1177/1759091419833502
Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R (2007) Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 146(1):9–16. https://doi.org/10.1016/j.neuroscience.2007.01.061
Beckel JM, Argall AJ, Lim JC, Xia J, Lu W, Coffey EE, Macarak EJ, Shahidullah M, Delamere NA, Zode GS, Sheffield VC, Shestopalov VI, Laties AM, Mitchell CH (2014) Mechanosensitive release of adenosine 5’-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia 62(9):1486–1501. https://doi.org/10.1002/glia.22695
Billaud M, Sandilos JK, Isakson BE (2012) Pannexin 1 in the regulation of vascular tone. Trends Cardiovasc Med 22(3):68–72. https://doi.org/10.1016/j.tcm.2012.06.014
Kauffenstein G, Furstenau CR, D’Orleans-Juste P, Sevigny J (2010) The ecto-nucleotidase NTPDase1 differentially regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation. Br J Pharmacol 159:576–585
Shestopalov VI, Slepak VZ (2014) Molecular pathways of pannexin1-mediated neurotoxicity. Front Physiol. https://doi.org/10.3389/fphys.2014.00023
Rink C, Khanna S (2011) Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid Redox Signal 14(10):1889–1903. https://doi.org/10.1089/ars.2010.3474
Kim Y, Davidson JO, Green CR, Nicholson LFB, O’Carroll SJ, Zhang J (2018) Connexins and Pannexins in cerebral ischemia. Biochim Biophys Acta Biomembr 1860(1):224–236. https://doi.org/10.1016/j.bbamem.2017.03.018
MacNee W (2005) Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2(4):258–291. https://doi.org/10.1513/pats.200504-045SR
Gombault A, Baron L, Couillin I (2013) ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol 3:414. https://doi.org/10.3389/fimmu.2012.00414
Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H (2014) Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med 5(8):927–946
Lusis AJ (2000) Atherosclerosis. Nature 407(6801):233–241. https://doi.org/10.1038/35025203
Makarenkova HP, Shah SB, Shestopalov VI (2018) The two faces of pannexins: new roles in inflammation and repair. J Inflamm Res 11:273–288. https://doi.org/10.2147/JIR.S128401
Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393(6686):648–659. https://doi.org/10.1038/31405
Wilen CB, Tilton JC, Doms RW (2012) HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2(8):a006866. https://doi.org/10.1101/cshperspect.a006866
Woodham AW, Skeate JG, Sanna AM et al (2016) Human immunodeficiency virus immune cell receptors, coreceptors, and cofactors: implications for prevention and treatment. AIDS Patient Care STDS 30(7):291–306. https://doi.org/10.1089/apc.2016.0100
Grove J, Marsh M (2011) The cell biology of receptor-mediated virus entry. J Cell Biol 195(7):1071–1082. https://doi.org/10.1083/jcb.201108131
Orellana JA, Velasquez S, Williams DW, Sáez JC, Berman JW, Eugenin EA (2013) Pannexin1 hemichannels are critical for HIV infection of human primary CD4+ T lymphocytes. J Leukoc Biol 94(3):399–407. https://doi.org/10.1189/jlb.0512249
Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC et al (2010) FGF1 induces ATP release from spinal astrocytes in culture and pannexin and connex in hemi channels. Proc Natl Acad Sci USA 107:22659–22664. https://doi.org/10.1073/pnas.1013793107
Iglesias RM, Spray DC (2012) Pannexin1 mediated ATP release provides signal transmission between Neuro2A cells. Neurochem Res 37:1355–1363. https://doi.org/10.1007/s11064-012-0720-6
Boyce AK, Kim MS, Wicki-Stordeur LE, Swayne LA (2015) ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem J 470(3):319–330. https://doi.org/10.1042/BJ20141551
Ardiles AO, Flores-Munoz C, Toro-Ayala G, Cardenas AM, Palacios AG, Munoz P, Fuenzalida M, Saez JC, Martinez AD (2014) Pannexin 1 regulates bidirectional hippocampal synaptic plasticity in adult mice. Front Cell Neurosci 8:326
Boucher J, Simonneau C, Denet G, Clarhaut J, Balandre A-C, Mesnil M, Cronier L, Monvoisin A (2018) Pannexin-1 in human lymphatic endothelial cells regulates lymphangiogenesis. Int J Mol Sci 19:1558
Celetti SJ, Cowan KN, Penuela S, Shao Q, Churko J, Laird DW (2010) Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J Cell Sci 123:1363–1372. https://doi.org/10.1242/jcs.056093
Dvoriantchikova G, Ivanov D, Panchin Y, Shestopalov VI (2006) Expression of pannexin family of proteins in the retina. FEBS Lett 580:2178–2182
Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M (2009) Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41(5):525–534. https://doi.org/10.1165/rcmb.2008-0367OC
Workman AD, Carey RM, Chen B et al (2017) CALHM1-mediated ATP release and ciliary beat frequency modulation in nasal epithelial cells. Sci Rep 7:6687. https://doi.org/10.1038/s41598-017-07221-9
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Cohen JJ (1991) Programmed cell death in the immune system. Adv Immunol 50:55–85
Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK (2003) Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell 4:587–598
Parrish AB, Freel CD, Kornbluth S (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 5(6):a008672. https://doi.org/10.1101/cshperspect.a008672
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC (2018) Neuronal cell death. Physiol Rev 98(2):813–880. https://doi.org/10.1152/physrev.00011.2017
Jan R, Chaudhry GE (2019) Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull 9(2):205–218. https://doi.org/10.15171/apb.2019.024
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157(5):1415–1430. https://doi.org/10.1016/S0002-9440(10)64779-7
Shoji KF, Sáez PJ, Harcha PA, Aguila HL, Sáez JC (2014) Pannexin1 channels act downstream of P2X 7 receptors in ATP-induced murine T-cell death. Channels. 8(2):142–156. https://doi.org/10.4161/chan.28122
Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett 581(3):483–488. https://doi.org/10.1016/j.febslet.2006.12.056
Boyce AKJ, Swayne LA (2017) P2X7 receptor cross-talk regulates ATP-induced pannexin 1 internalization. Biochem J 474(13):2133–2144. https://doi.org/10.1042/BCJ20170257
Swayne LA, Boyce AKJ (2017) Regulation of Pannexin 1 surface expression by extracellular ATP: potential implications for nervous system function in health and disease. Front Cell Neurosci 11:230. https://doi.org/10.3389/fncel.2017.00230
Crespo Yanguas S, Willebrords J, Johnstone SR et al (2017) Pannexin1 as mediator of inflammation and cell death. Biochim Biophys Acta Mol Cell Res 1864(1):51–61. https://doi.org/10.1016/j.bbamcr.2016.10.006
Wicki-Stordeur LE, Swayne LA (2013) Panx1 regulates neural stem and progenitor cell behaviours associated with cytoskeletal dynamics and interacts with multiple cytoskeletal elements. Cell Commun Signal 11:62. https://doi.org/10.1186/1478-811X-11-62
Malik A, Kanneganti TD (2018) Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol Rev 281(1):124–137. https://doi.org/10.1111/imr.12615
Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22(2):240–273. https://doi.org/10.1128/CMR.00046-08
Satoh T, Akira S (2016) Toll-like receptor signaling and its inducible proteins. Microbiol Spectr. 4(6):447. https://doi.org/10.1128/microbiolspec.MCHD-0040-2016
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Hayden MS, Ghosh S (2014) Regulation of NF-κB by TNF family cytokines. Semin Immunol 26(3):253–266. https://doi.org/10.1016/j.smim.2014.05.004
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13(6):397–411. https://doi.org/10.1038/nri3452
Davis BK, Wen H, Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29:707–735. https://doi.org/10.1146/annurev-immunol-031210-101405
Zheng D, Liwinski T, Elinav E (2020) Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov 6:36. https://doi.org/10.1038/s41421-020-0167-x
Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F (2017) The P2X7 receptor-interleukin-1 liaison. Front Pharmacol 8:123. https://doi.org/10.3389/fphar.2017.00123
Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S (2017) The P2X7 Receptor in infection and inflammation. Immunity 47(1):15–31. https://doi.org/10.1016/j.immuni.2017.06.020
Valdebenito S, Barreto A, Eugenin EA (2018) The role of connexin and pannexin containing channels in the innate and acquired immune response. Biochim Biophys Acta Biomembr 1860(1):154–165. https://doi.org/10.1016/j.bbamem.2017.05.015
Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G (2015) Caspase-11 requires the Pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43(5):923–932. https://doi.org/10.1016/j.immuni.2015.10.009
Bunderson-Schelvan M, Holian A, Hamilton RF Jr (2017) Engineered nanomaterial-induced lysosomal membrane permeabilization and anti-cathepsin agents. J Toxicol Environ Health B 20(4):230–248. https://doi.org/10.1080/10937404.2017.1305924
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 20(13):3328. https://doi.org/10.3390/ijms20133328
Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Koçak E, Sen ZD, Dalkara T (2013) Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339(6123):1092–1095. https://doi.org/10.1126/science.1231897 (Erratum.In:Science.2015Oct2;350(6256):aad5166.Erratumin:Science.2015Nov20;350(6263):921)
Author information
Authors and Affiliations
Contributions
EAB wrote the main manuscript and made figures; NS edited the manuscript and revised the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhat, E.A., Sajjad, N. Human Pannexin 1 channel: Insight in structure–function mechanism and its potential physiological roles. Mol Cell Biochem 476, 1529–1540 (2021). https://doi.org/10.1007/s11010-020-04002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-04002-3