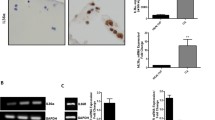
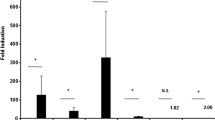
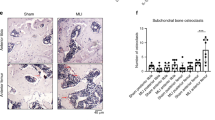
Abstract
Endocan is a circulating proteoglycan, involved in immunity, inflammation, and endothelial function. It has been recently suggested as a biomarker of inflammation, increased angiogenesis, and cancer. In vitro studies have shown that endocan expression could be upregulated by inflammatory cytokines and proangiogenic molecules. High endocan levels were also shown in arthritic joint tissues and particularly in sites characterized by severe inflammation. This study was performed to evaluate endocan expression in chondrocytes stimulated with IL-ß. mRNA and related protein production were measured for endocan, TNF-α, and IL-6. NF-kB activity was also evaluated. IL-1ß treatment induced a significant upregulation of both endocan and the inflammatory parameters as well as NF-kB activity. The treatment of chondrocytes with the specific NF-kB inhibitor before IL-1ß stimulation was able to reduce endocan and the inflammatory markers over-expression. The results of our study indicated that endocan is also expressed in human chondrocytes; furthermore, consistent with previous results in other cell types and tissues, IL-1ß-induced inflammatory response involves the expression of endocan through NF-kB activation. In this context, endocan seems to be an important inflammatory marker associated with the activation of NF-kB pathway.




Similar content being viewed by others
References
Scuruchi M, D'Ascola A, Avenoso A, Mandraffino GG, Campo SS, Campo GM (2019) Serglycin as part of IL-1beta induced inflammation in human chondrocytes. Arch Biochem Biophys 669:80–86. https://doi.org/10.1016/j.abb.2019.05.021
Iozzo RV, Schaefer L (2015) Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42:11–55. https://doi.org/10.1016/j.matbio.2015.02.003
Karamanos NK, Piperigkou Z, Theocharis AD, Watanabe H, Franchi M, Baud S, Brézillon S, Götte M, Passi A, Vigetti D, Ricard-Blum S, Sanderson RD, Neill T, Iozzo RV (2018) Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem Rev 118:9152–9232. https://doi.org/10.1021/acs.chemrev.8b00354
Manou D, Caon I, Bouris P, Triantaphyllidou IE, Giaroni C, Passi A, Karamanos NK, Vigetti D, Theocharis AD (2019) The complex interplay between extracellular matrix and cells in tissues. Methods Mol Biol 1952:1–20. https://doi.org/10.1007/978-1-4939-9133-4_1
Couchman JR (2010) Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol 26:89–114. https://doi.org/10.1146/annurev-cellbio-100109-104126
Iozzo RV (1998) Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 67:609–652. https://doi.org/10.1146/annurev.biochem.67.1.609
Kim SH, Turnbull J, Guimond S (2011) Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 209:139–151. https://doi.org/10.1530/joe-10-0377
Lambaerts K, Wilcox-Adelman SA, Zimmermann P (2009) The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr Opin Cell Biol 21:662–669. https://doi.org/10.1016/j.ceb.2009.05.002
Lortat-Jacob H (2009) The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol 19:543–548. https://doi.org/10.1016/j.sbi.2009.09.003
Mythreye K, Blobe GC (2009) Proteoglycan signaling co-receptors: roles in cell adhesion, migration and invasion. Cell Signal 21:1548–1558. https://doi.org/10.1016/j.cellsig.2009.05.001
Perrimon N, Bernfield M (2000) Specificities of heparan sulphate proteoglycans in developmental processes. Nature 404:725–728. https://doi.org/10.1038/35008000
Sarrazin S, Lamanna WC, Esko JD (2011) Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3. https://doi.org/10.1101/cshperspect.a004952
Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U (2002) Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer 2:521–528. https://doi.org/10.1038/nrc842
Spillmann D (2001) Heparan sulfate: anchor for viral intruders? Biochimie 83:811–817. https://doi.org/10.1016/s0300-9084(01)01290-1
Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK (2010) Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J 277:3904–3923. https://doi.org/10.1111/j.1742-4658.2010.07800.x
Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M (2006) Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta 1765:25–37. https://doi.org/10.1016/j.bbcan.2005.08.004
Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, Devos R, Tonnel AB (1996) ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem 271:20458–20464. https://doi.org/10.1074/jbc.271.34.20458
Béchard D, Gentina T, Delehedde M, Scherpereel A, Lyon M, Aumercier M, Vazeux R, Richet C, Degand P, Jude B, Janin A, Fernig DG, Tonnel AB, Lassalle P (2001) Endocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activity. J Biol Chem 276:48341–48349. https://doi.org/10.1074/jbc.M108395200
Fries E, Blom AM (2000) Bikunin–not just a plasma proteinase inhibitor. Int J Biochem Cell Biol 32:125–137. https://doi.org/10.1016/s1357-2725(99)00125-9
Stanley ER, Berg KL, Einstein DB, Lee PS, Yeung YG (1994) The biology and action of colony stimulating factor-1. Stem Cells 12(Suppl 1):15–24 discussion 25
Dieterich LC, Mellberg S, Langenkamp E, Zhang L, Zieba A, Salomäki H, Teichert M, Huang H, Edqvist PH, Kraus T, Augustin HG, Olofsson T, Larsson E, Söderberg O, Molema G, Pontén F, Georgii-Hemming P, Alafuzoff I, Dimberg A (2012) Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFβ2 in vascular abnormalization. J Pathol 228:378–390. https://doi.org/10.1002/path.4072
Maurage CA, Adam E, Minéo JF, Sarrazin S, Debunne M, Siminski RM, Baroncini M, Lassalle P, Blond S, Delehedde M (2009) Endocan expression and localization in human glioblastomas. J Neuropathol Exp Neurol 68:633–641. https://doi.org/10.1097/NEN.0b013e3181a52a7f
Gerritsen ME, Tomlinson JE, Zlot C, Ziman M, Hwang S (2003) Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol 140:595–610. https://doi.org/10.1038/sj.bjp.0705494
Kirwan RP, Leonard MO, Murphy M, Clark AF, O'Brien CJ (2005) Transforming growth factor-beta-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia 52:309–324. https://doi.org/10.1002/glia.20247
Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, Westholm JO, Komorowski J, Lassalle P, Cross MJ, Gerwins P (2007) Endocan is a VEGF-A and PI3K regulated gene with increased expression in human renal cancer. Exp Cell Res 313:1285–1294. https://doi.org/10.1016/j.yexcr.2007.01.021
Zhao X, Ramsey KE, Stephan DA, Russell P (2004) Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Invest Ophthalmol Vis Sci 45:4023–4034. https://doi.org/10.1167/iovs.04-0535
Abid MR, Yi X, Yano K, Shih SC, Aird WC (2006) Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res 72:136–145. https://doi.org/10.1016/j.mvr.2006.05.010
Lee W, Ku SK, Kim SW, Bae JS (2014) Endocan elicits severe vascular inflammatory responses in vitro and in vivo. J Cell Physiol 229:620–630. https://doi.org/10.1002/jcp.24485
Kim KS, Lee YA, Ji HI, Song R, Kim JY, Lee SH, Hong SJ, Yoo MC, Yang HI (2015) Increased expression of endocan in arthritic synovial tissues: effects of adiponectin on the expression of endocan in fibroblast-like synoviocytes. Mol Med Rep 11:2695–2702. https://doi.org/10.3892/mmr.2014.3057
Tseng CC, Chen YJ, Chang WA, Tsai WC, Ou TT, Wu CC, Sung WY, Yen JH, Kuo PL (2020) Dual role of chondrocytes in rheumatoid arthritis: the chicken and the egg. Int J Mol Sci 21. https://doi.org/10.3390/ijms21031071
Maldonado M, Nam J (2013) The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int 2013:284873. https://doi.org/10.1155/2013/284873
Avenoso A, D'Ascola A, Scuruchi M, Mandraffino G, Calatroni A, Saitta A, Campo S, Campo GM (2018) Hyaluronan in the experimental injury of the cartilage: biochemical action and protective effects. Inflamm Res 67:5–20. https://doi.org/10.1007/s00011-017-1084-9
Chow YY, Chin KY (2020) The role of inflammation in the pathogenesis of osteoarthritis. Mediat Inflamm 2020:8293921. https://doi.org/10.1155/2020/8293921
Tsunoda T, Takagi T (1999) Estimating transcription factor bindability on DNA. Bioinformatics 15:622–630. https://doi.org/10.1093/bioinformatics/15.7.622
Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, Meinhardt T, Prüss M, Reuter I, Schacherer F (2000) TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res 28:316–319. https://doi.org/10.1093/nar/28.1.316
Tanaka S (2019) RANKL is a therapeutic target of bone destruction in rheumatoid arthritis. F1000Res 8. https://doi.org/10.12688/f1000research.17296.1
Cai X, Zhang L, Wei W (2019) Regulatory B cells in inflammatory diseases and tumor. Int Immunopharmacol 67:281–286. https://doi.org/10.1016/j.intimp.2018.12.007
Di Benedetto P, Ruscitti P, Vadasz Z, Toubi E, Giacomelli R (2019) Macrophages with regulatory functions, a possible new therapeutic perspective in autoimmune diseases. Autoimmun Rev 18:102369. https://doi.org/10.1016/j.autrev.2019.102369
Hu XX, Wu YJ, Zhang J, Wei W (2019) T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. Int Immunopharmacol 70:428–434. https://doi.org/10.1016/j.intimp.2019.03.008
Bunte K, Beikler T (2019) Th17 cells and the IL-23/IL-17 Axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci 20. https://doi.org/10.3390/ijms20143394
Polasik K, Piotrowska E, Lipińska B, Witkowski JM, Bryl E, Tukaj S (2017) Vitamin D status in patients with rheumatoid arthritis: a correlation analysis with disease activity and progression, as well as serum IL-6 levels. Acta Biochim Pol 64:667–670. https://doi.org/10.18388/abp.2017_1636
Mateen S, Moin S, Shahzad S, Khan AQ (2017) Level of inflammatory cytokines in rheumatoid arthritis patients: correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS One 12:e0178879. https://doi.org/10.1371/journal.pone.0178879
Peck Y, Leom LT, Low PFP, Wang DA (2018) Establishment of an in vitro three-dimensional model for cartilage damage in rheumatoid arthritis. J Tissue Eng Regen Med 12:e237–e249. https://doi.org/10.1002/term.2399
Barksby HE, Hui W, Wappler I, Peters HH, Milner JM, Richards CD, Cawston TE, Rowan AD (2006) Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: implications for cartilage destruction and repair. Arthritis Rheum 54:540–550. https://doi.org/10.1002/art.21574
Otero M, Goldring MB (2007) Cells of the synovium in rheumatoid arthritis. Chondrocytes Arthritis Res Ther 9:220. https://doi.org/10.1186/ar2292
Campo GM, Avenoso A, D'Ascola A, Scuruchi M, Calatroni A, Campo S (2015) Beta-arrestin-2 negatively modulates inflammation response in mouse chondrocytes induced by 4-mer hyaluronan oligosaccharide. Mol Cell Biochem 399:201–208. https://doi.org/10.1007/s11010-014-2246-5
Grigoriu BD, Depontieu F, Scherpereel A, Gourcerol D, Devos P, Ouatas T, Lafitte JJ, Copin MC, Tonnel AB, Lassalle P (2006) Endocan expression and relationship with survival in human non-small cell lung cancer. Clin Cancer Res 12:4575–4582. https://doi.org/10.1158/1078-0432.Ccr-06-0185
Delehedde M, Devenyns L, Maurage CA, Vives RR (2013) Endocan in cancers: a lesson from a circulating dermatan sulfate proteoglycan. Int J Cell Biol 2013:705027. https://doi.org/10.1155/2013/705027
Yang J, Sheng S, Yang Q, Li L, Qin S, Yu S, Zhang X (2017) Endocan silencing induces programmed cell death in hepatocarcinoma. Oncol Lett 14:5333–5339. https://doi.org/10.3892/ol.2017.6857
Kang YH, Ji NY, Lee CI, Lee HG, Kim JW, Yeom YI, Kim DG, Yoon SK, Kim JW, Park PJ, Song EY (2011) ESM-1 silencing decreased cell survival, migration, and invasion and modulated cell cycle progression in hepatocellular carcinoma. Amino Acids 40:1003–1013. https://doi.org/10.1007/s00726-010-0729-6
Sun L, Sun C, Sun J, Yang W (2019) Downregulation of ENDOCAN in myeloid leukemia cells inhibits proliferation and promotes apoptosis by suppressing nuclear factor-κB activity. Mol Med Rep 19:3247–3254. https://doi.org/10.3892/mmr.2019.9969
Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Rigoglou S, Papavassiliou AG (2013) The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 45:2580–2584. https://doi.org/10.1016/j.biocel.2013.08.018
Olivotto E, Otero M, Marcu KB, Goldring MB (2015) Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open 1:e000061. https://doi.org/10.1136/rmdopen-2015-000061
Roudnicky F, Poyet C, Wild P, Krampitz S, Negrini F, Huggenberger R, Rogler A, Stöhr R, Hartmann A, Provenzano M, Otto VI, Detmar M (2013) Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis. Cancer Res 73:1097–1106. https://doi.org/10.1158/0008-5472.Can-12-1855
Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC (2002) Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1, (beta)ig-h3, and NrCAM. Microvasc Res 63:159–171. https://doi.org/10.1006/mvre.2001.2380
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scuruchi, M., D’Ascola, A., Avenoso, A. et al. Endocan, a novel inflammatory marker, is upregulated in human chondrocytes stimulated with IL-1 beta. Mol Cell Biochem 476, 1589–1597 (2021). https://doi.org/10.1007/s11010-020-04001-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-04001-4