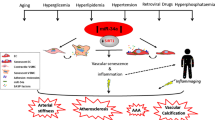
Abstract
MicroRNAs (miRNAs) are important molecules which implicated in various processes, such as differentiation, development, cell survival, cell apoptosis and also cell metabolism. Investigations over decades have revealed that various genes and signaling pathways are implicated in beginning and development of atherosclerosis, several miRNAs being involved in these dysregulated genes and pathways. miRNAs have provided new molecular vision in the context of atherosclerosis. miRNAs are considered as important regulators of cellular migration, differentiation, proliferation, lipid uptake and efflux, as well as cytokine production. Application of miRNAs as a biomarker in diagnosis, prognosis and even therapy is quiet exciting. Although animal researches showed promising results, still some practical difficulties and technical challenges need to be addressed before translation from researches into clinical practices. In this review, we present important data about three critical cells endothelial cell (EC), vascular smooth muscle cell (VSMC), and monocyte/macrophage and regulation of these cells through miRNAs. Furthermore, we discuss about the potential of miRNAs as a prognostic and diagnostic biomarkers, therapeutic opportunities and challenges, and also future perspective.

Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author. All data generated or analyzed during this study are included in this published article.
Abbreviations
- miRNAs:
-
MicroRNAs
- EC:
-
Endothelial cell
- VSMC:
-
Vascular smooth muscle cell
- SMC:
-
Smooth muscle cell
- ICAM:
-
Intracellular adhesion molecule
- VCAM:
-
Vascular adhesion molecule
- eNOS:
-
Endothelial nitric oxide synthase
- Dlk1:
-
Notch1 inhibitor delta-like 1 homolog
- PI3K:
-
Phosphatidylinositol kinase
- HDL-C:
-
High-density lipoprotein-cholesterol
- HBP1:
-
HMG box-transcriptional protein 1
- MIF:
-
Macrophage inhibitory factor
- BCL6:
-
B cell lymphoma 6
- SHIP-1:
-
Src homology 2 domain-containing inositol-5-phosphatase-1
- SOCS1:
-
Suppressor of cytokine signaling 1
- apoE:
-
Apolipoprotein E
- KLF2:
-
Krüppel-like factor 2
- PDCD4:
-
Programmed cell death 4
- ACE:
-
Angiotensin converting enzyme
- HCV:
-
Hepatitis C virus
References
Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278:483–493
Feinberg MW, Moore KJ (2016) MicroRNA regulation of atherosclerosis. Circ Res 118:703–720
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Ambros V (2004) The functions of animal microRNAs. Nature 431:350
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11:597
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835
Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20:515–524
Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP (2008) The impact of microRNAs on protein output. Nature 455:64
Friedman RC, Farh KK-H, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105
Samanta S, Balasubramanian S, Rajasingh S, Patel U, Dhanasekaran A, Dawn B, Rajasingh J (2016) MicroRNA: a new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc Med 26:407–419
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473:317
Staszel T, Zapala B, Polus A, Sadakierska-Chudy A, Kiec-Wilk B, Stepien E, Wybranska I, Chojnacka M, Dembinska-Kiec A (2011) Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn 121:361–366
Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, Zhou W, Xiong B, Zeng Q (2010) miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol 88:555–564
Fang Y, Shi C, Manduchi E, Civelek M, Davies PF (2010) MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci 107:13450–13455
Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE (2013) MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med 5:1017–1034
Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M (2014) MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20:368
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15:272–284
Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, Palmisano BT, Tabet F, Cui HL, Rye K-A (2014) MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci 111:14518–14523
Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, Li X, Shen N, Xiao D, Zhang H (2015) MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep 5:9401
Angelovich TA, Hearps AC, Jaworowski A (2015) Inflammation-induced foam cell formation in chronic inflammatory disease. Immunol Cell Biol 93:683
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13:709
Chang R, Ying W, Bazer F, Zhou B (2014) MicroRNAs control macrophage formation and activation: the inflammatory link between obesity and cardiovascular diseases. Cells 3:702–712
Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, Saura R, Kurosaka M, Kumagai S (2009) MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 60:1294–1304
Zhu D, Pan C, Li L, Bian Z, Lv Z, Shi L, Zhang J, Li D, Gu H, Zhang C-Y (2013) MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein α. J Allergy Clin Immunol 132(426–436):e8
Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M (2012) MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 126:S81–S90
Dorhoi A, Iannaccone M, Farinacci M, Faé KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf H-J, Oberbeck-Müller D, Jörg S (2013) MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Investig 123:4836–4848
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C (2007) MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 100:1579–1588
Wei Y, Nazari-Jahantigh M, Chan L, Zhu M, Heyll K, Corbalán-Campos J, Hartmann P, Thiemann A, Weber C, Schober A (2013) The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1-and microRNA-155-dependent pathway during atherosclerosis. Circulation 127:1609–1619
Zhang M, Wu J-F, Chen W-J, Tang S-L, Mo Z-C, Tang Y-Y, Li Y, Wang J-L, Liu X-Y, Peng J (2014) MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis 234:54–64
Wang Y-S, Zhou J, Hong K, Cheng X-S, Li Y-G (2015) MicroRNA-223 displays a protective role against cardiomyocyte hypertrophy by targeting cardiac troponin I-interacting kinase. Cell Physiol Biochem 35:1546–1556
Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, Wang C (2009) MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res 83:131–139
Tian F-J, An L-N, Wang G-K, Zhu J-Q, Li Q, Zhang Y-Y, Zeng A, Zou J, Zhu R-F, Han X-S (2014) Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc Res 103:100–110
Ramirez CM, Dávalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, Suárez Y, Fernández-Hernando C (2011) MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol 31:2707–2714
Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA (2014) MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol 114:304878
Goedeke L, Rotllan N, Canfrán-Duque A, Aranda JF, Ramírez CM, Araldi E, Lin C-S, Anderson NN, Wagschal A, De Cabo R (2015) MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat Med 21:1280
Sun D, Zhang J, Xie J, Wei W, Chen M, Zhao X (2012) MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS Lett 586:1472–1479
Kim J, Yoon H, Ramírez CM, Lee S-M, Hoe H-S, Fernández-Hernando C, Kim J (2012) MiR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression. Exp Neurol 235:476–483
Marquart TJ, Allen RM, Ory DS, Baldán Á (2010) miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci 107:12228–12232
Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Näär AM (2010) MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328:1566–1569
Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C (2010) MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328:1570–1573
de Aguiar VT, Tarling E, Kim T, Civelek M, Baldan A, Esau C, Edwards P (2013) MicroRNA-144 regulates hepatic ABCA1 and plasma HDL following activation of the nuclear receptor FXR. Circ Res 112:300648
Wanschel A, Zavadil J, Castrillo A, Jungsu K, Suárez Y, Aranda CJF, Cirera-Salinas D, Araldi E, Salerno A, Bonito AC (2013) Control of cholesterol metabolism and plasma HDL levels by miRNA-144
Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S (2012) A novel regulator of macrophage activation: miR-223 in obesity associated adipose tissue inflammation. Circulation 125:2892–2903
Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang C-Y, Zen K (2012) Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol 4:341–343
Yang J, Zhang Z, Chen C, Liu Y, Si Q, Chuang T, Li N, Gomez-Cabrero A, Reisfeld R, Xiang R (2014) MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene 33:3014
Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K (2015) MicroRNA-33–dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Investig 125:4334–4348
Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G (2013) MicroRNA let-7c regulates macrophage polarization. J Immunol 190:6542–6549
Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, Thannickal VJ, Abraham E, Liu G (2013) miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem 288:35428–35436
Wang Z, Brandt S, Medeiros A, Wang S, Wu H, Dent A, Serezani CH (2015) MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS ONE 10:e0115855
Lu S, Gao Y, Huang X, Wang X (2014) Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int J Biol Sci 10:415
Saha B, Bruneau JC, Kodys K, Szabo G (2015) Alcohol-induced miR-27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol 194:3079–3087
Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, Alexaki VI, Helms M, Kondili E, Soennichsen B (2014) Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol 192:394–406
Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED (2013) IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS ONE 8:e81774
Sun H-X, Zeng D-Y, Li R-T, Pang R-P, Yang H, Hu Y-L, Zhang Q, Jiang Y, Huang L-Y, Tang Y-B (2012) Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 60(6):1407–1414
Yao R, Ma Y-L, Liang W, Li H-H, Ma Z-J, Yu X, Liao Y-H (2012) MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS ONE 7:e46082
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J (2012) MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Investig 122:4190–4202
Liu G, Friggeri A, Yang Y, Park Y-J, Tsuruta Y, Abraham E (2009) miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci 106:15819–15824
Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’learyRuanJohnsonChenO’neill JJQDSYLA (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11:141
Li K, Ching D, Luk FS, Raffai RL (2015) Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circ Res 114:305844
Manoharan P, Basford JE, Pilcher-Roberts R, Neumann J, Hui DY, Lingrel JB (2014) Reduced levels of microRNAs miR-124a and miR-150 are associated with increased proinflammatory mediator expression in Krüppel-like factor 2 (KLF2)-deficient macrophages. J Biol Chem 289:31638–31646
Doran AC, Meller N, McNamara CA (2008) Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol 28:812–819
Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21:628
Davis BN, Hilyard AC, Lagna G, Hata A (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454:56
Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A (2009) Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 284:3728–3738
Liu X, Cheng Y, Yang J, Xu L, Zhang C (2012) Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol 52:245–255
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee T-H, Miano JM, Ivey KN, Srivastava D (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460:705
Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN (2009) MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 23(18):2166–2178
Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J (2009) The knockout of miR-143 and-145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 16:1590
Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, Braun T (2009) Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Investig 119:2634–2647
Cheng Y, Liu X, Yang J, Lin Y, Xu D-Z, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C (2009) MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 105:158–166
Faruq O, Vecchione A (2015) microRNA: diagnostic perspective. Front Med 2:51
Sayed ASM, Xia K, Salma U, Yang T, Peng J (2014) Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ 23:503–510
Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM (2010) MiR423-5p as a circulating biomarker for heart failure. Circ Res 106:1035–1039
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M (2010) Circulating microRNAs in patients with coronary artery disease novelty and significance. Circ Res 107:677–684
Boštjančič E, Zidar N, Štajer D, Glavač D (2010) MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology 115:163–169
Broderick JA, Zamore PD (2011) MicroRNA therapeutics. Gene Ther 18:1104
Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S (2012) Inhibition of microRNA function by antimiR oligonucleotides. Silence 3:1
Van Rooij E, Olson EN (2012) MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 11:860
Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U (2008) LNA-mediated microRNA silencing in non-human primates. Nature 452:896
Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X (2011) Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 478:404
Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E (2010) Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 28:172
Dowling JE, Wald G (1981) Proceedings of the National Academy of Sciences of the United States of America. Nutr Rev 39:135–138
Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J (2004) Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432:173
Ebert MS, Neilson JR, Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4:721
Kluiver J, Slezak-Prochazka I, Smigielska-Czepiel K, Halsema N, Kroesen B-J, van den Berg A (2012) Generation of miRNA sponge constructs. Methods 58:113–117
Sun X, He S, Wara A, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK (2014) Systemic delivery of microRNA-181b inhibits nuclear factor-κb activation, vascular inflammation, and atherosclerosis in apolipoprotein E–deficient mice. Circ Res 114:32–40
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423
Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM (2012) MicroRNA-181b regulates NF-κB–mediated vascular inflammation. J Clin Investig 122:1973–1990
Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309:1577–1581
Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H (2010) Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327:198–201
Ling H, Fabbri M, Calin GA (2013) MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 12:847
Beg MS, Brenner AJ, Sachdev J, Borad M, Kang Y-K, Stoudemire J, Smith S, Bader AG, Kim S, Hong DS (2017) Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig New Drugs 35:180–188
Ni J, Sun Y, Liu Z (2019) The potential of stem cells and stem cell-derived exosomes in treating cardiovascular diseases. J Cardiovasc Transl Res 12:51–61
Araujo CL, Quintero IB, Kipar A, Herrala AM, Pulkka AE, Saarinen L, Hautaniemi S, Vihko P (2014) Prostatic acid phosphatase is the main acid phosphatase with 5’-ectonucleotidase activity in the male mouse saliva and regulates salivation. Am J Physiol Cell Physiol 306:C1017–C1027. https://doi.org/10.1152/ajpcell.00062.2014
Wahid F, Shehzad A, Khan T, Kim YY (2010) MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta Mol Cell Res 1803:1231–1243
Acknowledgements
The authors are grateful of Deputy of Research from Neyshabur University of Medical Science.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Consent for publication
All authors read the manuscript and consent for its publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabaei, S., Tabaee, S.S. Implications for MicroRNA involvement in the prognosis and treatment of atherosclerosis. Mol Cell Biochem 476, 1327–1336 (2021). https://doi.org/10.1007/s11010-020-03992-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03992-4