Abstract
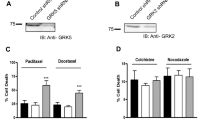
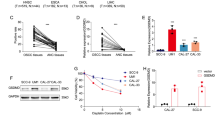
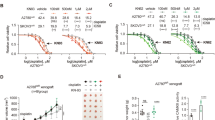
G protein-coupled receptor kinases (GRKs), in addition to their role in modulating signal transduction mechanisms associated with activated G protein-coupled receptors (GPCRs), can also interact with many non-GPCR proteins to mediate cellular responses to chemotherapeutics. The rationale for this study is based on the presumption that GRK2 modulates the responses of cancer cells to the chemotherapeutic cisplatin. In this report, we show that GRK2 modulates the responses of cancer cells to cisplatin. Cervical cancer HeLa cells stably transfected with GRK2 shRNA, to decrease GRK2 protein expression, show increased sensitivity to cisplatin. Of interest, these cells also show increased accumulation of NADPH, associating with decreased NADP buildup, at low concentrations of cisplatin tested. These changes in NADPH and NADP levels are also observed in the breast cancer MDA MB 231 cells, which has lower endogenous GRK2 protein expression levels, but not BT549, a breast cancer cell line with higher GRK2 protein expression. This effect of NADPH accumulation may be associated with a decrease in NADPH oxidase 4 (NOX4) protein expression, which is found to correlate with GRK2 protein expression in cancer cells—a relationship which mimics that observed in cardiomyocytes. Furthermore, like in cardiomyocytes, GRK2 and NOX4 interact to form complexes in cancer cells. Collectively, these results suggest that GRK2 interacts with NOX4 to modify cisplatin sensitivity in cancer cells and may also factor into the success of cisplatin-based regimens.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author [CS] upon reasonable request.
Abbreviations
- GRK:
-
G protein-coupled receptor kinase
- GPCRs:
-
G protein-coupled receptors
- HDAC:
-
Histone deacetylase
- NOX4:
-
NADPH oxidase 4
References
Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV (2012) G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther 133:40–69. https://doi.org/10.1016/j.pharmthera.2011.08.001
Yu S, Sun L, Jiao Y, Lee LTO (2018) The role of G protein-coupled receptor kinases in cancer. Int J Biol Sci 14:189–203. https://doi.org/10.7150/ijbs.22896
Chen X, Zhu H, Yuan M, Fu J, Zhou Y, Ma L (2010) G-protein-coupled receptor kinase 5 phosphorylates p53 and inhibits DNA damage-induced apoptosis. J Biol Chem 285:12823–12830. https://doi.org/10.1074/jbc.M109.094243
Pathania AS, Ren X, Mahdi MY, Shackleford GM, Erdreich-Epstein A (2019) GRK2 promotes growth of medulloblastoma cells and protects them from chemotherapy-induced apoptosis. Sci Rep 9:13902. https://doi.org/10.1038/s41598-019-50157-5
Penela P, Rivas V, Salcedo A, Mayor F (2010) G protein-coupled receptor kinase 2 (GRK2) modulation and cell cycle progression. Proc Natl Acad Sci USA 107:1118–1123. https://doi.org/10.1073/pnas.0905778107
Lagman J, Sayegh P, Lee CS, Sulon SM, Jacinto AZ, Sok V, Peng N, Alp D, Benovic JL, So CH (2019) G protein-coupled receptor kinase 5 modifies cancer cell resistance to paclitaxel. Mol Cell Biochem 461:103–118. https://doi.org/10.1007/s11010-019-03594-9
So CH, Michal AM, Mashayekhi R, Benovic JL (2012) G protein-coupled receptor kinase 5 phosphorylates nucleophosmin and regulates cell sensitivity to polo-like kinase 1 inhibition. J Biol Chem 287:17088–17099. https://doi.org/10.1074/jbc.M112.353854
W-y S, Wu J-j, W-t P, J-c S, Wei W (2018) The role of G protein-coupled receptor kinases in the pathology of malignant tumors. Acta Pharmacol Sin 39:1699–1705. https://doi.org/10.1038/s41401-018-0049-z
Martini JS, Raake P, Vinge LE, DeGeorge BR, DeGeorge B, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ (2008) Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci USA 105:12457–12462. https://doi.org/10.1073/pnas.0803153105
Michal AM, So CH, Beeharry N, Shankar H, Mashayekhi R, Yen TJ, Benovic JL (2012) G Protein-coupled receptor kinase 5 is localized to centrosomes and regulates cell cycle progression. J Biol Chem 287:6928–6940. https://doi.org/10.1074/jbc.M111.298034
So CH, Michal A, Komolov KE, Luo J, Benovic JL (2013) G protein-coupled receptor kinase 2 (GRK2) is localized to centrosomes and mediates epidermal growth factor-promoted centrosomal separation. Mol Biol Cell 24:2795–2806. https://doi.org/10.1091/mbc.E13-01-0013
Franco A, Sorriento D, Gambardella J, Pacelli R, Prevete N, Procaccini C, Matarese G, Trimarco B, Iaccarino G, Ciccarelli M (2018) GRK2 moderates the acute mitochondrial damage to ionizing radiation exposure by promoting mitochondrial fission/fusion. Cell Death Dis 4:25. https://doi.org/10.1038/s41420-018-0028-7
Manfredi LH, Ang J, Peker N, Dagda RK, McFarlane C (2019) G protein-coupled receptor kinase 2 regulates mitochondrial bioenergetics and impairs myostatin-mediated autophagy in muscle cells. Am J Phys Cell Phys 317:C674–c686. https://doi.org/10.1152/ajpcell.00516.2018
Sorriento D, Fusco A, Ciccarelli M, Rungi A, Anastasio A, Carillo A, Dorn GW, Trimarco B, Iaccarino G (2013) Mitochondrial G protein coupled receptor kinase 2 regulates proinflammatory responses in macrophages. FEBS Lett 587:3487–3494. https://doi.org/10.1016/j.febslet.2013.09.002
Gold JI, Martini JS, Hullmann J, Gao E, Chuprun JK, Lee L, Tilley DG, Rabinowitz JE, Bossuyt J, Bers DM, Koch WJ (2013) Nuclear translocation of cardiac G protein-coupled receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PLoS One 8:e57324. https://doi.org/10.1371/journal.pone.0057324
Sato PY, Chuprun JK, Grisanti LA, Woodall MC, Brown BR, Roy R, Traynham CJ, Ibetti J, Lucchese AM, Yuan A, Drosatos K, Tilley DG, Gao E, Koch WJ (2018) Restricting mitochondrial GRK2 post-ischemia confers cardioprotection by reducing myocyte death and maintaining glucose oxidation. Sci Signal 11. https://doi.org/10.1126/scisignal.aau0144
Luo J, Benovic JL (2003) G protein-coupled receptor kinase interaction with Hsp90 mediates kinase maturation. J Biol Chem 278:50908–50914. https://doi.org/10.1074/jbc.M307637200
Theccanat T, Philip JL, Razzaque AM, Ludmer N, Li J, Xu X, Akhter SA (2016) Regulation of cellular oxidative stress and apoptosis by G protein-coupled receptor kinase-2; The role of NADPH oxidase 4. Cell Signal 28:190–203. https://doi.org/10.1016/j.cellsig.2015.11.013
Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, Doetsch PW (2013) Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 8:e81162. https://doi.org/10.1371/journal.pone.0081162
Bai L, Mao R, Wang J, Ding L, Jiang S, Gao C, Kang H, Chen X, Sun X, Xu J (2015) ERK1/2 promoted proliferation and inhibited apoptosis of human cervical cancer cells and regulated the expression of c-Fos and c-Jun proteins. Med Oncol 32:57. https://doi.org/10.1007/s12032-015-0490-5
Li M, Liu X, He Y, Zheng Q, Wang M, Wu Y, Zhang Y, Wang C (2017) Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2/Nox2 signal pathway. Eur J Pharmacol 797:124–133. https://doi.org/10.1016/j.ejphar.2017.01.027
Lee JW, Lee J, Moon EY (2014) HeLa human cervical cancer cell migration is inhibited by treatment with dibutyryl-cAMP. Anticancer Res 34:3447–3455
Li Z, Yang Z, Lapidus RG, Liu X, Cullen KJ, Dan HC (2015) IKK phosphorylation of NF-κB at serine 536 contributes to acquired cisplatin resistance in head and neck squamous cell cancer. Am J Cancer Res 5(10):3098–3110
Prabhakaran P, Hassiotou F, Blancafort P, Filgueira L (2013) Cisplatin induces differentiation of breast cancer cells. Front Oncol 3:134. https://doi.org/10.3389/fonc.2013.00134
Tahara H, Matsuda S, Yamamoto Y, Yoshizawa H, Fujita M, Katsuoka Y, Kasahara T (2017) High-content image analysis (HCIA) assay has the highest correlation with direct counting cell suspension compared to the ATP, WST-8 and Alamar blue assays for measurement of cytotoxicity. J Pharmacol Toxicol Methods 88:92–99. https://doi.org/10.1016/j.vascn.2017.08.003
Weyemi U, Redon CE, Parekh PR, Dupuy C, Bonner WM (2013) NADPH Oxidases NOXs and DUOXs as putative targets for cancer therapy. Anti Cancer Agents Med Chem 13:502–514
Kim H-J, Lee J-H, Kim S-J, Oh GS, Moon H-D, Kwon K-B, Park C, Park BH, Lee H-K, Chung S-Y, Park R, So H-S (2010) Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci 30:3933. https://doi.org/10.1523/JNEUROSCI.6054-09.2010
Li S, Zhang X, Zhang R, Liang Z, Liao W, Du Z, Gao C, Liu F, Fan Y, Hong H (2017) Hippo pathway contributes to cisplatin resistant-induced EMT in nasopharyngeal carcinoma cells. Cell Cycle 16:1601–1610. https://doi.org/10.1080/15384101.2017.1356508
Chen SH, Chang JY (2019) New insights into mechanisms of cisplatin resistance: from tumor cell to microenvironment. Int J Mol Sci 20. https://doi.org/10.3390/ijms20174136
Sampson N, Brunner E, Weber A, Puhr M, Schafer G, Szyndralewiez C, Klocker H (2018) Inhibition of Nox4-dependent ROS signaling attenuates prostate fibroblast activation and abrogates stromal-mediated protumorigenic interactions. Int J Cancer 143:383–395. https://doi.org/10.1002/ijc.31316
Chang G, Chen L, Lin HM, Lin Y, Maranchie JK (2012) Nox4 inhibition enhances the cytotoxicity of cisplatin in human renal cancer cells. J Exp Ther Oncol 10:9–18
Shono T, Yokoyama N, Uesaka T, Kuroda J, Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, Miyamoto K, Akashi K, Iwaki T, Sumimoto H, Sasaki T (2008) Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer 123:787–792. https://doi.org/10.1002/ijc.23569
Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS (2004) Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279:34643–34654. https://doi.org/10.1074/jbc.M400078200
Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovskaya AS (2007) NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 133:1637–1648. https://doi.org/10.1053/j.gastro.2007.08.022
Meng XM, Ren GL, Gao L, Yang Q, Li HD, Wu WF, Huang C, Zhang L, Lv XW, Li J (2018) NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab Investig 98:63–78. https://doi.org/10.1038/labinvest.2017.120
Yamaura M, Mitsushita J, Furuta S, Kiniwa Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, Saida T, Kamata T (2009) NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res 69:2647–2654. https://doi.org/10.1158/0008-5472.Can-08-3745
Hsieh CH, Wu CP, Lee HT, Liang JA, Yu CY, Lin YJ (2012) NADPH oxidase subunit 4 mediates cycling hypoxia-promoted radiation resistance in glioblastoma multiforme. Free Radic Biol Med 53:649–658. https://doi.org/10.1016/j.freeradbiomed.2012.06.009
Salmeen A, Park BO, Meyer T (2010) The NADPH oxidases NOX4 and DUOX2 regulate cell cycle entry via a p53-dependent pathway. Oncogene 29:4473–4484. https://doi.org/10.1038/onc.2010.200
Zeng C, Wu Q, Wang J, Yao B, Ma L, Yang Z, Li J, Liu B (2016) NOX4 supports glycolysis and promotes glutamine metabolism in non-small cell lung cancer cells. Free Radic Biol Med 101:236–248. https://doi.org/10.1016/j.freeradbiomed.2016.10.500
Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, Dupuy C (2010) Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer 17:27–37. https://doi.org/10.1677/erc-09-0175
Yu W, Chen Y, Dubrulle J, Stossi F, Putluri V, Sreekumar A, Putluri N, Baluya D, Lai SY, Sandulache VC (2018) Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci Rep 8:4306. https://doi.org/10.1038/s41598-018-22640-y
Naka K, Muraguchi T, Hoshii T, Hirao A (2008) Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal 10:1883–1894. https://doi.org/10.1089/ars.2008.2114
Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, Stevenson DE, Walborg EF Jr (1998) The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect 106(Suppl 1):289–295. https://doi.org/10.1289/ehp.98106s1289
Ushio-Fukai M, Nakamura Y (2008) Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett 266:37–52. https://doi.org/10.1016/j.canlet.2008.02.044
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183. https://doi.org/10.1016/j.redox.2015.01.002
Yuan X, Wang B, Yang L, Zhang Y (2018) The role of ROS-induced autophagy in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 42:306–312. https://doi.org/10.1016/j.clinre.2018.01.005
Zhang J, Yang L, Xiang X, Li Z, Qu K, Li K (2018) A panel of three oxidative stress-related genes predicts overall survival in ovarian cancer patients received platinum-based chemotherapy. Aging (Albany NY) 10:1366–1379. https://doi.org/10.18632/aging.101473
Lu H, Sun C, Zhou T, Zhou B, Guo E, Shan W, Xia M, Li K, Weng D, Meng L, Xu X, Hu J, Ma D, Chen G (2016) HSP27 knockdown increases cytoplasmic p21 and cisplatin sensitivity in ovarian carcinoma cells. Oncol Res 23:119–128. https://doi.org/10.3727/096504015x14496932933656
Zhang J, Lei W, Chen X, Wang S, Qian W (2018) Oxidative stress response induced by chemotherapy in leukemia treatment. Mol Clin Oncol 8:391–399. https://doi.org/10.3892/mco.2018.1549
Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, Chen Y, Cang H, Li H, Shi G, Cheng J, Tang X, Yi J (2009) SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J 28:2748–2762. https://doi.org/10.1038/emboj.2009.210
Sorriento D, Ciccarelli M, Cipolletta E, Trimarco B, Iaccarino G (2016) “Freeze, don’t move”: how to arrest a suspect in heart failure – a review on available GRK2 inhibitors. Front Cardiovasc Med 3:48. https://doi.org/10.3389/fcvm.2016.00048
Acknowledgements
We like to thank Dr. Murat Alp and Deniz Alp for critical review of the manuscript. This work was partly funded by the Roseman University of Health Sciences College of Pharmacy Intramural Grant.
Funding
The funding of this work was provided by Roseman University of Health Sciences College of Pharmacy and the Roseman University of Health Sciences College of Pharmacy Intramural Grant Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that there are no competing interests associated with the manuscript.
Consent for publication
The Author grants the Publisher the sole and exclusive license of the full copyright in the Contribution.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ammon, J.C., Valls, D., Eldemerdash, M. et al. G protein-coupled receptor kinase 2 modifies the cellular reaction to cisplatin through interactions with NADPH oxidase 4. Mol Cell Biochem 476, 1505–1516 (2021). https://doi.org/10.1007/s11010-020-03969-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03969-3