Abstract
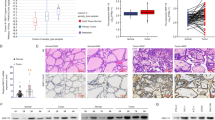
Kin17 DNA and RNA binding protein (Kin17) is an extremely conserved nuclear protein that is almost expressed in every type of mammal cells. Recently, Kin17 has been implicated into the regulation of tumorigenesis of diverse human cancers. However, its functions in thyroid cancer (TC) are still largely unexplored. Kin17 mRNA and protein level were tested by qRT-PCR and western blot, respectively. Effects of Kin17 on TC cell proliferation were estimated by colony formation assay and flow cytometry analysis in vitro as well as by in vivo tumor growth experiment. TC cell migratory and invasive capacities were assessed via wound-healing and transwell experiments. Epithelial–mesenchymal transition (EMT)-related proteins (E-cadherin and N-cadherin) and p38 MAPAK signaling pathway-related proteins (p-p38, p38, Cyclin D1, and p27) were examined via western blot. Kin17 was remarkably increased in TC tissue samples and cell lines at both mRNA and protein levels compared to normal tissue and control cell line. Knockdown of Kin17 obviously repressed TC cell proliferation, arrested cell cycle, and inhibited TC cell migration and invasion in vitro, while overexpression of Kin17 produced opposite effects. Kin17 knockdown suppressed p38 MAPK signaling pathway, while Kin17 overexpression activated this pathway. Treatment of p38 agonist (p79350) abolished the repressive effects of sh-Kin17 on TC cell proliferation, migration, and invasion, as well as on p38 pathway. Kin17 knockdown was also found to enhance the sensitivity of Doxorubicin of TC cells. In addition, Kin17 knockdown in vivo also markedly repressed TC tumor growth and p38 pathway. Kin17 functioned as an oncogene of TC by activating p38 MAPK signaling pathway.








Similar content being viewed by others
Change history
09 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11010-020-04018-9
Abbreviations
- TC:
-
Thyroid cancer
- FTC:
-
Follicular thyroid cancer
- MTC:
-
Medullary thyroid carcinoma
- PDTC:
-
Poorly differentiated thyroid cancer
- ATC:
-
Anaplastic thyroid cancer
- MAPK:
-
Mitogen-activated protein kinase
- NSCLC:
-
Non-small cell lung cancer
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Baloch ZW, LiVolsi VA (2018) Special types of thyroid carcinoma. Histopathology 72:40–52. https://doi.org/10.1111/his.13348
Azar FK, Lee SL, Rosen JE (2015) Medullary thyroid cancer: an update for surgeons. Am Surg 81:1–8
Massimino M, Evans DB, Podda M, Spinelli C, Collini P, Pizzi N, Bleyer A (2018) Thyroid cancer in adolescents and young adults. Pediatr Blood Cancer 65:e27025. https://doi.org/10.1002/pbc.27025
Roman BR, Morris LG, Davies L (2017) The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes 24:332–336. https://doi.org/10.1097/MED.0000000000000359
Miccoli P, Bakkar S (2017) Surgical management of papillary thyroid carcinoma: an overview. Updates Surg 69:145–150. https://doi.org/10.1007/s13304-017-0449-5
Barbet J, Campion L, Kraeber-Bodere F, Chatal JF, Group GTES (2005) Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 90:6077–6084. https://doi.org/10.1210/jc.2005-0044
Schmidbauer B, Menhart K, Hellwig D, Grosse J (2017) Differentiated thyroid cancer-treatment: state of the art. Int J Mol Sci. https://doi.org/10.3390/ijms18061292
Cloutier P, Lavallee-Adam M, Faubert D, Blanchette M, Coulombe B (2014) Methylation of the DNA/RNA-binding protein Kin17 by METTL22 affects its association with chromatin. J Proteomics 100:115–124. https://doi.org/10.1016/j.jprot.2013.10.008
Tissier A, Kannouche P, Mauffrey P, Allemand I, Frelat G, Devoret R, Angulo JF (1996) Molecular cloning and characterization of the mouse Kin17 gene coding for a Zn-finger protein that preferentially recognizes bent DNA. Genomics 38:238–242. https://doi.org/10.1006/geno.1996.0623
Kannouche P, Mauffrey P, Pinon-Lataillade G, Mattei MG, Sarasin A, Daya-Grosjean L, Angulo JF (2000) Molecular cloning and characterization of the human KIN17 cDNA encoding a component of the UVC response that is conserved among metazoans. Carcinogenesis 21:1701–1710. https://doi.org/10.1093/carcin/21.9.1701
Miccoli L, Frouin I, Novac O, Di Paola D, Harper F, Zannis-Hadjopoulos M, Maga G, Biard DS, Angulo JF (2005) The human stress-activated protein kin17 belongs to the multiprotein DNA replication complex and associates in vivo with mammalian replication origins. Mol Cell Biol 25:3814–3830. https://doi.org/10.1128/MCB.25.9.3814-3830.2005
Gao X, Liu Z, Zhong M, Wu K, Zhang Y, Wang H, Zeng T (2019) Knockdown of DNA/RNA-binding protein KIN17 promotes apoptosis of triple-negative breast cancer cells. Oncol Lett 17:288–293. https://doi.org/10.3892/ol.2018.9597
Zhang Y, Gao H, Gao X, Huang S, Wu K, Yu X, Yuan K, Zeng T (2017) Elevated expression of Kin17 in cervical cancer and its association with cancer cell proliferation and invasion. Int J Gynecol Cancer 27:628–633. https://doi.org/10.1097/IGC.0000000000000928
Kou WZ, Xu SL, Wang Y, Wang LW, Wang L, Chai XY, Hua QL (2014) Expression of Kin17 promotes the proliferation of hepatocellular carcinoma cells in vitro and in vivo. Oncol Lett 8:1190–1194. https://doi.org/10.3892/ol.2014.2244
Zeng T, Gao H, Yu P, He H, Ouyang X, Deng L, Zhang Y (2011) Up-regulation of kin17 is essential for proliferation of breast cancer. PLoS ONE 6:e25343. https://doi.org/10.1371/journal.pone.0025343
Cuenda A, Rousseau S (2007) p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773:1358–1375. https://doi.org/10.1016/j.bbamcr.2007.03.010
Cuadrado A, Nebreda AR (2010) Mechanisms and functions of p38 MAPK signalling. Biochem J 429:403–417. https://doi.org/10.1042/BJ20100323
Kim WG, Choi HJ, Kim TY, Shong YK, Kim WB (2014) The effect of 5-aminoimidazole-4-carboxamide-ribonucleoside was mediated by p38 mitogen activated protein kinase signaling pathway in FRO thyroid cancer cells. Korean J Intern Med 29:474–481. https://doi.org/10.3904/kjim.2014.29.4.474
Pomerance M, Quillard J, Chantoux F, Young J, Blondeau JP (2006) High-level expression, activation, and subcellular localization of p38-MAP kinase in thyroid neoplasms. J Pathol 209:298–306. https://doi.org/10.1002/path.1975
Aschebrook-Kilfoy B, Schechter RB, Shih YC, Kaplan EL, Chiu BC, Angelos P, Grogan RH (2013) The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomark Prev 22:1252–1259. https://doi.org/10.1158/1055-9965.EPI-13-0242
Zhang Y, Huang S, Gao H, Wu K, Ouyang X, Zhu Z, Yu X, Zeng T (2017) Upregulation of KIN17 is associated with non-small cell lung cancer invasiveness. Oncol Lett 13:2274–2280. https://doi.org/10.3892/ol.2017.5707
Ray A, Cleary MP (2017) The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev 38:80–97. https://doi.org/10.1016/j.cytogfr.2017.11.002
Grossi V, Peserico A, Tezil T, Simone C (2014) p38alpha MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol 20:9744–9758. https://doi.org/10.3748/wjg.v20.i29.9744
Gupta J, Nebreda AR (2015) Roles of p38alpha mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J 282:1841–1857. https://doi.org/10.1111/febs.13250
Han SE, Park CH, Nam-Goong IS, Kim YI, Kim ES (2019) Anticancer effects of baicalein in FRO thyroid cancer cells through the up-regulation of ERK/p38 MAPK and Akt pathway. In Vivo 33:375–382. https://doi.org/10.21873/invivo.11484
Funding
This work is supported by the National Natural Science Foundation of China (No. 81660294) and The Youth Fund of Department of Education of Jiangxi Province (No. GJJ180146).
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: Y-XL; study concepts: Y-XL, Q-GJ; study design: Q-GJ; definition of intellectual content: C-FX; literature research: C-FX; clinical studies: Q-GJ; experimental studies: Y-XL, Q-GJ; data acquisition: Y-XL, Q-GJ; data analysis: C-FX; statistical analysis: Q-GJ; manuscript preparation: C-FX; manuscript editing: Q-GJ; manuscript review: Y-XL.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no commercial or other associations that might pose a conflict of interest.
Ethical approval
Manipulates in this study were approved by the Ethics Committee of The First Affiliated Hospital of NanChang University, and written informed consent was provided to each participant. Human TC cell lines (K1, SW579, 8505C, TPC1, and BCPAP) and human thyroid follicular epithelial cells (Nthy-ori 3–1) were purchased from the American Type Culture Collection (ATCC, USA).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, QG., Xiong, CF. & Lv, YX. Kin17 facilitates thyroid cancer cell proliferation, migration, and invasion by activating p38 MAPK signaling pathway. Mol Cell Biochem 476, 727–739 (2021). https://doi.org/10.1007/s11010-020-03939-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03939-9