Abstract
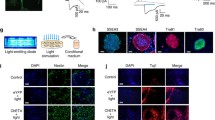
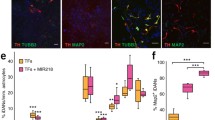
Parkinson’s disease (PD) is a disorder characterized by a progressive loss of the dopaminergic neurons in the substantia nigra and a depletion of the neurotransmitter dopamine in the striatum. Our published results indicate that fasciculation and elongation protein zeta-1 (FEZ1) plays a role in the astrocyte-mediated protection of dopamine neurons and regulation of the neuronal microenvironment during the progression of PD. In this study, we examined the effects of engrafted type-2 astrocytes (T2As) with high expression of FEZ1 on the improvement of the symptoms and functional reconstruction of PD rats. T2As were stereotactically transplanted into the striatum of rats with PD induced by 6-hydroxydopamine (6-OHDA). An examination of apomorphine (APO)-induced rotations was performed to evaluate dopamine neuron damage and motor functions. Remarkably, the grafted cells survived in the lesion environment for six weeks or longer after implantation. In addition, the transplantation of T2As decrease the average velocity and the duration time of the APO-induced rotations, and increase the actuation time, as measured in the rotation behavioural tests. In the substantia nigra, the transplantation of T2As reduced the PD-induced GFAP, TH and FEZ1 downregulation. The grafted cells exclusively migrated to other regions near the injection site in the striatum and differentiated into GFAP+ astrocytes or TH+ neurons. Furthermore, by detecting monoamine neurotransmitters through high-performance liquid chromatography, we found that the nigrostriatal pathway had been repaired to some extent. Taken together, these results suggest that engrafted T2As with high expression of FEZ1 improved the symptoms and functional reconstruction of PD rats, providing a theoretical basis for FEZ1 as a potential target and engraftment of T2As as a therapeutic strategy in the treatment of PD.





Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease
- FEZ1:
-
Fasciculation and elongation protein zeta-1
- T2As:
-
Type-2 astrocytes
- 6-OHDA:
-
6-Hydroxydopamine
- APO:
-
Apomorphine
- DA:
-
Dopamine
- 5-HT:
-
5-Hydroxytryptamine
- DOPAC:
-
Dihydroxy-phenyl acetic acid
- HVA:
-
Homovanillic acid
- O-2A:
-
Oligodendrocyte-type-2 astrocyte progenitor
- ACSF:
-
Artificial cerebrospinal fluid
References
Raza C, Anjum R, Shakeel NUA (2019) Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci 226:77–90. https://doi.org/10.1016/j.lfs.2019.03.057
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68:384–386. https://doi.org/10.1212/01.wnl.0000247740.47667.03
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. https://doi.org/10.1073/pnas.95.11.6469
Bandres-Ciga S, Ruz C, Barrero FJ, Escamilla-Sevilla F, Pelegrina J, Vives F, Duran R (2017) Structural genomic variations and Parkinson’s disease. Minerva Med 108:438–447. https://doi.org/10.23736/S0026-4806.17.05246-6
Fiandaca MS, Bankiewicz KS, Federoff HJ (2012) Gene therapy for the treatment of Parkinson’s disease: the nature of the biologics expands the future indications. Pharmaceuticals 5:553–590. https://doi.org/10.3390/ph5060553
Rothlind JC, York MK, Carlson K, Luo P, Marks WJ Jr, Weaver FM, Stern M, Follett K, Reda D, Group CSPS (2015) Neuropsychological changes following deep brain stimulation surgery for Parkinson’s disease: comparisons of treatment at pallidal and subthalamic targets versus best medical therapy. J Neurol Neurosurg Psychiatry 86:622–629. https://doi.org/10.1136/jnnp-2014-308119
Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS (2019) Emerging therapies in Parkinson disease—repurposed drugs and new approaches. Nat Rev Neurol 15:204–223. https://doi.org/10.1038/s41582-019-0155-7
Picazio S, Ponzo V, Caltagirone C, Brusa L, Koch G (2018) Dysfunctional inhibitory control in Parkinson’s disease patients with levodopa-induced dyskinesias. J Neurol 265:2088–2096. https://doi.org/10.1007/s00415-018-8945-1
Chaudhuri KR, Rizos A, Sethi KD (2013) Motor and nonmotor complications in Parkinson’s disease: an argument for continuous drug delivery? J Neural Transm 120:1305–1320. https://doi.org/10.1007/s00702-013-0981-5
Vernon AC (2009) Mice with reduced vesicular monoamine storage content display nonmotor features of Parkinson’s disease. J Neurosci 29:12842–12844. https://doi.org/10.1523/JNEUROSCI.4156-09.2009
Lelieveld IM, Muller ML, Bohnen NI, Koeppe RA, Chervin RD, Frey KA, Albin RL (2012) The role of serotonin in sleep disordered breathing associated with Parkinson disease: a correlative [11C]DASB PET imaging study. PLoS ONE 7:e40166. https://doi.org/10.1371/journal.pone.0040166
Lu X, Fu X, Zhai W, Xia C (2015) Behavioral evaluation and changes in levels of monoamine neurotransmitter within the nigrostriatal pathway in rat models of Parkinson’s disease. Chin J Clinicalanatomy 33:182–188
Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K (1999) Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein. J Cell Biol 144:403–411
Ikuta J, Maturana A, Fujita T, Okajima T, Tatematsu K, Tanizawa K, Kuroda S (2007) Fasciculation and elongation protein zeta-1 (FEZ1) participates in the polarization of hippocampal neuron by controlling the mitochondrial motility. Biochem Biophys Res Commun 353:127–132. https://doi.org/10.1016/j.bbrc.2006.11.142
Honda A, Miyoshi K, Baba K, Taniguchi M, Koyama Y, Kuroda S, Katayama T, Tohyama M (2004) Expression of fasciculation and elongation protein zeta-1 (FEZ1) in the developing rat brain. Brain Res Mol Brain Res 122:89–92. https://doi.org/10.1016/j.molbrainres.2003.11.020
He J, Liu J, Zhang Z, Sun M, Zhu T, Xia C (2009) Expression of fasciculation and elongation protein zeta-1 (FEZ1) in cultured rat neonatal astrocytes. Mol Cell Biochem 325:159–167. https://doi.org/10.1007/s11010-009-0030-8
Jeong JY, Einhorn Z, Mercurio S, Lee S, Lau B, Mione M, Wilson SW, Guo S (2006) Neurogenin1 is a determinant of zebrafish basal forebrain dopaminergic neurons and is regulated by the conserved zinc finger protein Tof/Jezl. Proc Natl Acad Sci USA 103:5143–5148. https://doi.org/10.1073/pnas.0600337103
Sakae N, Yamasaki N, Kitaichi K, Fukuda T, Yamada M, Yoshikawa H, Hiranita T, Tatsumi Y, Kira J, Yamamoto T, Miyakawa T, Nakayama KI (2008) Mice lacking the schizophrenia-associated protein FEZ1 manifest hyperactivity and enhanced responsiveness to psychostimulants. Hum Mol Genet 17:3191–3203. https://doi.org/10.1093/hmg/ddn215
Sun YY, Zhang Y, Sun XP, Liu TY, Liu ZH, Chen G, Xia CL (2014) Fasciculation and elongation protein zeta-1 (FEZ1) expression in reactive astrocytes in a rat model of Parkinson’s disease. Neuropathol Appl Neurobiol 40:164–176. https://doi.org/10.1111/nan.12077
Mena MA, de Bernardo S, Casarejos MJ, Canals S, Rodriguez-Martin E (2002) The role of astroglia on the survival of dopamine neurons. Mol Neurobiol 25:245–263. https://doi.org/10.1385/MN:25:3:245
Dervan AG, Meshul CK, Beales M, McBean GJ, Moore C, Totterdell S, Snyder AK, Meredith GE (2004) Astroglial plasticity and glutamate function in a chronic mouse model of Parkinson’s disease. Exp Neurol 190:145–156. https://doi.org/10.1016/j.expneurol.2004.07.004
Raff MCMR, Noble M (1983) A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303:390–396
Ffrench-Constant C, Raff MC (1986) The oligodendrocyte-type-2 astrocyte cell lineage is specialized for myelination. Nature 323:335–338. https://doi.org/10.1038/323335a0
Chunpeng L, Ye Z, Chun-Lin X, Hui S, Jing Z, Zhifang L (2007) The expression of nestin and SSEA-1 in the rat type-2 astrocytes. Acta Anat Sin 38:153–157
Kondo T, Raff M (2000) Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289:1754–1757
Kondo T, Raff M (2004) Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev 18:2963–2972. https://doi.org/10.1101/gad.309404
Paxinos GWC (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego
Gullapalli RR, Demirel MC, Butler PJ (2008) Molecular dynamics simulations of DiI-C18(3) in a DPPC lipid bilayer. Phys Chem Chem Phys 10:3548–3560. https://doi.org/10.1039/b716979e
Matsubayashi Y, Iwai L, Kawasaki H (2008) Fluorescent double-labeling with carbocyanine neuronal tracing and immunohistochemistry using a cholesterol-specific detergent digitonin. J Neurosci Methods 174:71–81. https://doi.org/10.1016/j.jneumeth.2008.07.003
Chen X, Lu M, Ma N, Yin G, Cui C, Zhao S (2016) Dynamic tracking of injected mesenchymal stem cells after myocardial infarction in rats: a serial 7T MRI study. Stem Cells Int 2016:4656539. https://doi.org/10.1155/2016/4656539
Lin Z, Dodd CA, Filipov NM (2013) Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol Teratol 39:26–35. https://doi.org/10.1016/j.ntt.2013.06.002
Zhang W, He H, Song H, Zhao J, Li T, Wu L, Zhang X, Chen J (2016) Neuroprotective effects of salidroside in the MPTP mouse model of Parkinson’s disease: involvement of the PI3K/Akt/GSK3beta pathway. Parkinsons Dis 2016:9450137. https://doi.org/10.1155/2016/9450137
Le F, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B (2010) Glia as a turning point in the therapeutic strategy of Parkinson’s disease. CNS Neurol Disord Drug Targets 9:349–372
Grothe C, Timmer M (2007) The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Res Rev 54:80–91. https://doi.org/10.1016/j.brainresrev.2006.12.001
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Saavedra A, Baltazar G, Santos P, Carvalho CM, Duarte EP (2006) Selective injury to dopaminergic neurons up-regulates GDNF in substantia nigra postnatal cell cultures: role of neuron-glia crosstalk. Neurobiol Dis 23:533–542. https://doi.org/10.1016/j.nbd.2006.04.008
Petrova PS, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire A, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW (2004) Discovering novel phenotype-selective neurotrophic factors to treat neurodegenerative diseases. Prog Brain Res 146:168–183
Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160
Honig MG, Hume RI (1986) Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol 103:171–187
Sharma G, Vijayaraghavan S (2001) Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA 98:4148–4153. https://doi.org/10.1073/pnas.071540198
Acknowledgements
This work was supported by National Natural Science Foundation of China (81701316), by the Jiangsu Province Health Research Project (No. YG201307), and by Suzhou Science and Technology Plan (sys2018025, SYS2019025).
Author information
Authors and Affiliations
Contributions
The work was performed and accomplished by all authors. CX are the principal investigators for the project design, the interpretation of results. YS, XL, XF, YEZ, YAZ, JL and LZ performed experiments, analysed the data, made the figures and drafted the manuscript. YS, CX and XL wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors claim that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Y., Lu, Xj., Fu, X. et al. Engrafted primary type-2 astrocytes improve the recovery of the nigrostriatal pathway in a rat model of Parkinson's disease. Mol Cell Biochem 476, 619–631 (2021). https://doi.org/10.1007/s11010-020-03931-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03931-3