Abstract
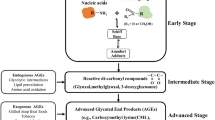
Advanced glycation end products (AGEs) are formed as a result of non-enzymatic reaction between the free reducing sugars and proteins, lipids, or nucleic acids. AGEs are predominantly synthesized during chronic hyperglycemic conditions or aging. AGEs interact with their receptor RAGE and activate various sets of genes and proteins of the signal transduction pathway. Accumulation of AGEs and upregulated expression of RAGE is associated with various pathological conditions including diabetes, cardiovascular diseases, neurodegenerative disorders, and cancer. The role of AGE-RAGE signaling has been demonstrated in the progression of various types of cancer and other pathological disorders. The expression of RAGE increases manifold during cancer progression. The activation of AGE-RAGE signaling also perturbs the cellular redox balance and modulates various cell death pathways. The programmed cell death signaling often altered during the progression of malignancies. The cellular reprogramming of AGE-RAGE signaling with cell death machinery during tumorigenesis is interesting to understand the complex signaling mechanism of cancer cells. The present review focus on multiple molecular paradigms relevant to cell death particularly Apoptosis, Autophagy, and Necroptosis that are considerably influenced by the AGE-RAGE signaling in the cancer cells. Furthermore, the review also attempts to shed light on the provenience of AGE-RAGE signaling on oxidative stress and consequences of cell survival mechanism of cancer cells.




Similar content being viewed by others
Abbreviations
- AGE:
-
Advanced glycation end products
- Akt:
-
Protein kinase B
- DAMPs:
-
Damage-associated molecular patterns
- ERK:
-
Extracellular-signal-regulated kinase
- HMGB1:
-
High‐mobility group box 1
- JAK:
-
Janus kinase
- MAPK:
-
Mitogen-activated protein kinase
- NF-kB:
-
Nuclear factor kappa B
- NOX:
-
NADPH oxidase
- Nrf-2:
-
Nuclear factor erythroid 2-related factor 2
- PAMPs:
-
Pathogen-associated molecular patterns
- PI3K:
-
Phosphoinositide 3-kinases
- RAGE:
-
Receptor of advanced glycation endproducts
- ROS:
-
Reactive oxygen species
- STAT-3:
-
Signal transducer and activator of transcription 3
- TLRs:
-
Toll-like receptors
References
Chen J-H, Lin X, Bu C, Zhang X (2018) Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr Metab 15:72
Chhipa AS, Borse SP, Baksi R, Lalotra S, Nivsarkar M (2019) Targeting receptors of advanced glycation end products (RAGE): preventing diabetes induced cancer and diabetic complications. Pathol Res Pract 215:152643. https://doi.org/10.1016/j.prp.2019.152643
Singh VP, Bali A, Singh N, Jaggi AS (2014) Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 18:1–14. https://doi.org/10.4196/kjpp.2014.18.1.1
Ramasamy R, Yan SF, Schmidt AM (2011) Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci 1243:88–102. https://doi.org/10.1111/j.1749-6632.2011.06320.x
Senatus LM, Schmidt AM (2017) The AGE-RAGE axis: implications for age-associated arterial diseases. Front Gent 8:187
Soman S, Raju R, Sandhya VK, Advani J, Khan AA, Harsha HC, Prasad TSK, Sudhakaran PR, Pandey A, Adishesha PK (2013) A multicellular signal transduction network of AGE/RAGE signaling. J Cell Commun Signal 7:19–23. https://doi.org/10.1007/s12079-012-0181-3
Hudson BI, Lippman ME (2018) Targeting RAGE signaling in inflammatory disease. Annu Rev Med 69:349–364. https://doi.org/10.1146/annurev-med-041316-085215
Clavel J (2007) Progress in the epidemiological understanding of gene-environment interactions in major diseases: cancer. CR Biol 330:306–317. https://doi.org/10.1016/j.crvi.2007.02.012
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA (2014) Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014:149185
Blaylock RL (2015) Cancer microenvironment, inflammation and cancer stem cells: a hypothesis for a paradigm change and new targets in cancer control. Surg Neurol Int 6:92
Liou G-Y, Storz P (2010) Reactive oxygen species in cancer. Free Radical Res 44:479–496
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30:1073–1081
Multhoff G, Molls M, Radons J (2012) Chronic inflammation in cancer development. Front Immunol 2:98–98. https://doi.org/10.3389/fimmu.2011.00098
Ryu TY, Park J, Scherer PE (2014) Hyperglycemia as a risk factor for cancer progression. Diab Metab J 38:330–336
Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, Bierhaus A, Lotze MT, Zeh HJ (2010) The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ 17:666–676
El-Far AH, Sroga G, Jaouni SKA, Mousa SA (2020) Role and mechanisms of RAGE-ligand complexes and RAGE-inhibitors in cancer progression. Int J Mol Sci. https://doi.org/10.3390/ijms21103613
Riehl A, Németh J, Angel P, Hess J (2009) The receptor RAGE: bridging inflammation and cancer. Cell Commun Signal 7:12–12. https://doi.org/10.1186/1478-811X-7-12
Nasser MW, Ahirwar DK, Ganju RK (2016) RAGE: a novel target for breast cancer growth and metastasis. Oncoscience 3:52–53. https://doi.org/10.18632/oncoscience.294
Rojas A, Figueroa H, Morales E (2010) Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis 31:334–341
Khan MI, Rath S, Adhami VM and Mukhtar H (2018) Hypoxia driven glycation: mechanisms and therapeutic opportunities. Semin Cancer Biol 49:75–82. https://doi.org/10.1016/j.semcancer.2017.05.008
Kang R, Hou W, Zhang Q, Chen R, Lee Y, Bartlett D, Lotze M, Tang D, Zeh H (2014) RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death Dis 5:e1480
El-Far A (2016) The role of receptors for advanced glycation end product in pancreatic carcinogenesis. Pancreat Disord Ther 6:166
Chen M-C, Chen K-C, Chang G-C, Lin H, Wu C-C, Kao W-H, Teng C-LJ, Hsu S-L, Yang T-Y (2020) RAGE acts as an oncogenic role and promotes the metastasis of human lung cancer. Cell Death Dise 11:1–13
Matou-Nasri S, Sharaf H, Wang Q, Almobadel N, Rabhan Z, Al-Eidi H, Yahya WB, Trivilegio T, Ali R, Al-Shanti N (2017) Biological impact of advanced glycation endproducts on estrogen receptor-positive MCF-7 breast cancer cells. Biochim Biophys Acta BBA 1863:2808–2820
Xu X, Abuduhadeer X, Zhang W, Li T, Gao H, Wang Y (2013) Knockdown of RAGE inhibits growth and invasion of gastric cancer cells. Eur J Histochem 57:e36
Swami P, Radhakrishnan P, Crawford A, Patil P, Shin S, Caffrey T, Grunkemeyer J, O’connell K, Hollingsworth M, Leclerc E (2019) Combination of RAGE inhibitors and gemcitabine impedes tumor growth by reducing autophagy and facilitating apoptosis in pancreatic cancer. FASEP J 33:674.19
Kwak T, Drews-Elger K, Ergonul A, Miller P, Braley A, Hwang G, Zhao D, Besser A, Yamamoto Y, Yamamoto H (2017) Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene 36:1559–1572
Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H (2005) Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate 64:92–100
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta BBA 1863:2977–2992
Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21:1–21. https://doi.org/10.1038/s41580-020-0230-3
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757
Palanissami G, Paul SF (2018) RAGE and its ligands: molecular interplay between glycation, inflammation, and hallmarks of cancer—a review. Horm Cancer 9:295–325
Tan AL, Forbes JM, Cooper ME (2007) AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol 27:130–143. https://doi.org/10.1016/j.semnephrol.2007.01.006
Nowotny K, Jung T, Höhn A, Weber D, Grune T (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5:194–222
Warboys CM, Toh H-B, Fraser PA (2009) Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci 50:1319–1328
Sun Y, Peng Z (2009) Programmed cell death and cancer. Postgrad Med J 85:134–140
Gewies A (2003) Introduction to apoptosis. Apo Rev 3:1–26
Denton D, Kumar S (2019) Autophagy-dependent cell death. Cell Death Differ 26:605–616
Choi ME, Price DR, Ryter SW, Choi AM (2019) Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight 4:e128834
Jo Y, Choi N, Kim K, Koo H-J, Choi J, Kim HN (2018) Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics 8:5259
Kim S, Kwon J (2013) COMP-Ang1 inhibits apoptosis as well as improves the attenuated osteogenic differentiation of mesenchymal stem cells induced by advanced glycation end products. Biochim Biophys Acta 1830:4928–4934. https://doi.org/10.1016/j.bbagen.2013.06.035
Barlovic DP, Thomas MC, Jandeleit-Dahm K (2010) Cardiovascular disease: what's all the AGE/RAGE about? Cardiovasc Hematol Disord Drug Targets 10:7–15
Zhan Y, Sun HL, Chen H, Zhang H, Sun J, Zhang Z, Cai DH (2012) Glucagon-like peptide-1 (GLP-1) protects vascular endothelial cells against advanced glycation end products (AGEs)-induced apoptosis. Med Sci Monit 18:286–291. https://doi.org/10.12659/msm.883207
Kim J, Kim KM, Kim CS, Sohn E, Lee YM, Jo K, Kim JS (2012) Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free Radic Biol Med 53:357–365. https://doi.org/10.1016/j.freeradbiomed.2012.04.030
Gao Y, Wake H, Morioka Y, Liu K, Teshigawara K, Shibuya M, Zhou J, Mori S, Takahashi H, Nishibori M (2017) Phagocytosis of advanced glycation end products (AGEs) in macrophages induces cell apoptosis. Oxid Med Cell Longev 2017:8419035
Strasser A, O'Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245. https://doi.org/10.1146/annurev.biochem.69.1.217
Boulanger E, Wautier MP, Wautier JL, Boval B, Panis Y, Wernert N, Danze PM, Dequiedt P (2002) AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int 61:148–156. https://doi.org/10.1046/j.1523-1755.2002.00115.x
Xie J, Mendez JD, Mendez-Valenzuela V, Aguilar-Hernandez MM (2013) Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal 25:2185–2197. https://doi.org/10.1016/j.cellsig.2013.06.013
Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, Parker TG (2010) S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ Res 106:93–101. https://doi.org/10.1161/CIRCRESAHA.109.195834
Wang P, Xing Y, Chen C, Chen Z, Qian Z (2016) Advanced glycation end-product (AGE) induces apoptosis in human retinal ARPE-19 cells via promoting mitochondrial dysfunction and activating the Fas-FasL signaling. Biosci Biotechnol Biochem 80:250–256. https://doi.org/10.1080/09168451.2015.1095065
Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyama I (2003) High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum 48:971–981. https://doi.org/10.1002/art.10859
Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF (2003) Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101:2652–2660. https://doi.org/10.1182/blood-2002-05-1300
Lecomte M, Denis U, Ruggiero D, Lagarde M, Wiernsperger N (2004) Involvement of caspase-10 in advanced glycation end-product-induced apoptosis of bovine retinal pericytes in culture. Biochim Biophys Acta 1689:202–211. https://doi.org/10.1016/j.bbadis.2004.03.010
Denis U, Lecomte M, Paget C, Ruggiero D, Wiernsperger N, Lagarde M (2002) Advanced glycation end-products induce apoptosis of bovine retinal pericytes in culture: involvement of diacylglycerol/ceramide production and oxidative stress induction. Free Radic Biol Med 33:236–247. https://doi.org/10.1016/s0891-5849(02)00879-1
Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang CY, Zen K (2014) Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol 192:437–446. https://doi.org/10.4049/jimmunol.1301790
Sun C, Liang C, Ren Y, Zhen Y, He Z, Wang H, Tan H, Pan X, Wu Z (2009) Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Res Cardiol 104:42–49. https://doi.org/10.1007/s00395-008-0738-8
Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A (2014) Role of advanced glycation end products in cellular signaling. Redox Biol 2:411–429. https://doi.org/10.1016/j.redox.2013.12.016
Alikhani M, Maclellan CM, Raptis M, Vora S, Trackman PC, Graves DT (2007) Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Cell Physiol 292:C850–C856. https://doi.org/10.1152/ajpcell.00356.2006
Kumar S, Boehm J, Lee JC (2003) p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2:717–726. https://doi.org/10.1038/nrd1177
Liu J, Lin A (2005) Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15:36–42. https://doi.org/10.1038/sj.cr.7290262
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282:11221–11229. https://doi.org/10.1074/jbc.M611871200
Huang HL, Hsieh MJ, Chien MH, Chen HY, Yang SF, Hsiao PC (2014) Glabridin mediate caspases activation and induces apoptosis through JNK1/2 and p38 MAPK pathway in human promyelocytic leukemia cells. PLoS ONE 9:e98943. https://doi.org/10.1371/journal.pone.0098943
Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT (2007) Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone 40:345–353. https://doi.org/10.1016/j.bone.2006.09.011
Shi L, Yu X, Yang H, Wu X (2013) Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. PLoS ONE 8:e66781. https://doi.org/10.1371/journal.pone.0066781
Liu BF, Miyata S, Hirota Y, Higo S, Miyazaki H, Fukunaga M, Hamada Y, Ueyama S, Muramoto O, Uriuhara A, Kasuga M (2003) Methylglyoxal induces apoptosis through activation of p38 mitogen-activated protein kinase in rat mesangial cells. Kidney Int 63:947–957. https://doi.org/10.1046/j.1523-1755.2003.00829.x
Sekido H, Suzuki T, Jomori T, Takeuchi M, Yabe-Nishimura C, Yagihashi S (2004) Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem Biophys Res Commun 320:241–248. https://doi.org/10.1016/j.bbrc.2004.05.159
Alikhani Z, Alikhani M, Boyd CM, Nagao K, Trackman PC, Graves DT (2005) Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem 280:12087–12095. https://doi.org/10.1074/jbc.M406313200
Kowluru RA (2005) Effect of advanced glycation end products on accelerated apoptosis of retinal capillary cells under in vitro conditions. Life Sci 76:1051–1060. https://doi.org/10.1016/j.lfs.2004.10.017
Min C, Kang E, Yu SH, Shinn SH, Kim YS (1999) Advanced glycation end products induce apoptosis and procoagulant activity in cultured human umbilical vein endothelial cells. Diab Res Clin Pract 46:197–202
Riuzzi F, Sorci G, Donato R (2006) The amphoterin (HMGB1)/receptor for advanced glycation end products (RAGE) pair modulates myoblast proliferation, apoptosis, adhesiveness, migration, and invasiveness. Functional inactivation of RAGE in L6 myoblasts results in tumor formation in vivo. J Biol Chem 281:8242–8253. https://doi.org/10.1074/jbc.M509436200
Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV (2013) Many faces of DAMPs in cancer therapy. Cell Death Dis 4:e631. https://doi.org/10.1038/cddis.2013.156
Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT (2010) HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 29:5299–5310. https://doi.org/10.1038/onc.2010.261
Zhang X, Chen Y, Cai G, Li X, Wang D (2017) Carnosic acid induces apoptosis of hepatocellular carcinoma cells via ROS-mediated mitochondrial pathway. Chem Biol Interact 277:91–100. https://doi.org/10.1016/j.cbi.2017.09.005
Tse AK, Cao HH, Cheng CY, Kwan HY, Yu H, Fong WF, Yu ZL (2014) Indomethacin sensitizes TRAIL-resistant melanoma cells to TRAIL-induced apoptosis through ROS-mediated upregulation of death receptor 5 and downregulation of survivin. J Invest Dermatol 134:1397–1407. https://doi.org/10.1038/jid.2013.471
Lo MC, Chen MH, Lee WS, Lu CI, Chang CR, Kao SH, Lee HM (2015) Nepsilon-(carboxymethyl) lysine-induced mitochondrial fission and mitophagy cause decreased insulin secretion from beta-cells. Am J Physiol Endocrinol Metab 309:E829–E839. https://doi.org/10.1152/ajpendo.00151.2015
Yu Y, Wang L, Delguste F, Durand A, Guilbaud A, Rousselin C, Schmidt AM, Tessier F, Boulanger E, Neviere R (2017) Advanced glycation end products receptor RAGE controls myocardial dysfunction and oxidative stress in high-fat fed mice by sustaining mitochondrial dynamics and autophagy-lysosome pathway. Free Radic Biol Med 112:397–410. https://doi.org/10.1016/j.freeradbiomed.2017.08.012
Mao YX, Cai WJ, Sun XY, Dai PP, Li XM, Wang Q, Huang XL, He B, Wang PP, Wu G, Ma JF, Huang SB (2018) RAGE-dependent mitochondria pathway: a novel target of silibinin against apoptosis of osteoblastic cells induced by advanced glycation end products. Cell Death Dis 9:674. https://doi.org/10.1038/s41419-018-0718-3
Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ (2014) The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene 33:567–577. https://doi.org/10.1038/onc.2012.631
Xu L, Fan Q, Wang X, Zhao X, Wang L (2016) Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis 7:e2445. https://doi.org/10.1038/cddis.2016.322
Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M, Makita Z (2002) Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J Biol Chem 277:20309–20315. https://doi.org/10.1074/jbc.M202634200
Hung LF, Huang KY, Yang DH, Chang DM, Lai JH, Ho LJ (2010) Advanced glycation end products induce T cell apoptosis: involvement of oxidative stress, caspase and the mitochondrial pathway. Mech Ageing Dev 131:682–691. https://doi.org/10.1016/j.mad.2010.09.005
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ (2009) RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7:17
Dhumale SS, Waghela BN, Pathak C (2015) Quercetin protects necrotic insult and promotes apoptosis by attenuating the expression of RAGE and its ligand HMGB1 in human breast adenocarcinoma cells. IUBMB Life 67:361–373
Kang R, Tang D, Lotze MT, Zeh I, Herbert J (2011) RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy 7:442–444
Zhang Q-Y, Wu L-Q, Zhang T, Han Y-F, Lin X (2015) Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol Rep 33:1630–1638
Cheng P, Dai W, Wang F, Lu J, Shen M, Chen K, Li J, Zhang Y, Wang C, Yang J (2014) Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1–RAGE and AKT pathways. Biochem Biophys Res Commun 443:1162–1168
Elangovan I, Thirugnanam S, Chen A, Zheng G, Bosland MC, Kajdacsy-Balla A, Gnanasekar M (2012) Targeting receptor for advanced glycation end products (RAGE) expression induces apoptosis and inhibits prostate tumor growth. Biochem Biophys Res Commun 417:1133–1138
Lan C-Y, Chen S-Y, Kuo C-W, Lu C-C, Yen G-C (2019) Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food Drug Anal 27:887–896
Kang R, Tang D, Loze MT, Zeh I, Herbert J (2011) Apoptosis to autophagy switch triggered by the MHC class III-encoded receptor for advanced glycation endproducts (RAGE). Autophagy 7:91–93
Recouvreux MV, Commisso C (2017) Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front Endocrinol (Lausanne) 8:261. https://doi.org/10.3389/fendo.2017.00261
Schmukler E, Kloog Y, Pinkas-Kramarski R (2014) Ras and autophagy in cancer development and therapy. Oncotarget 5:577–586. https://doi.org/10.18632/oncotarget.1775
Yun CW, Lee SH (2018) The roles of autophagy in cancer. Int J Mol Sci. https://doi.org/10.3390/ijms19113466
Daskalaki I, Gkikas I, Tavernarakis N (2018) Hypoxia and selective autophagy in cancer development and therapy. Front Cell Dev Biol 6:104. https://doi.org/10.3389/fcell.2018.00104
Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, Morgan MJ, Mirsky DM, Handler MH, Foreman NK, Thorburn A (2014) Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors. Cancer Discov 4:773–780. https://doi.org/10.1158/2159-8290.CD-14-0049
Cho SW, Na W, Choi M, Kang SJ, Lee S-G, Choi CY (2017) Autophagy inhibits cell death induced by the anti-cancer drug morusin. Am J Cancer Res 7:518–530
Lane JD, Carroll B, Hewitt G, Korolchuk VI (2013) Autophagy and ageing: implications for age-related neurodegenerative diseases. Essays Biochem 55:119–131
Hu P, Lai D, Lu P, Gao J, He H (2012) ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med 29:613–618
Kang R, Tang D, Livesey KM, Schapiro NE, Lotze MT, Zeh HJ 3rd (2011) The receptor for advanced glycation end-products (RAGE) protects pancreatic tumor cells against oxidative injury. Antioxid Redox Signal 15:2175–2184. https://doi.org/10.1089/ars.2010.3378
Piperi C, Adamopoulos C, Papavassiliou AG (2017) Potential of glycative stress targeting for cancer prevention. Cancer Lett 390:153–159. https://doi.org/10.1016/j.canlet.2017.01.020
Verma N, Manna SK (2016) Advanced glycation end products (AGE) potently induce autophagy through activation of RAF protein kinase and nuclear factor kappaB (NF-kappaB). J Biol Chem 291:1481–1491. https://doi.org/10.1074/jbc.M115.667576
He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE (2011) Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis 21:277–285
Ko SY, Ko HA, Shieh TM, Chi TC, Chen HI, Chen YT, Yu YH, Yang SH, Chang SS (2017) Advanced glycation end products influence oral cancer cell survival via Bcl-xl and Nrf-2 regulation in vitro. Oncol Lett 13:3328–3334
Gao W, Zhou Z, Liang B, Huang Y, Yang Z, Chen Y, Zhang L, Yan C, Wang J, Lu L, Wen Z, Xian S, Wang L (2018) Inhibiting receptor of advanced glycation end products attenuates pressure overload-induced cardiac dysfunction by preventing excessive autophagy. Front Physiol 9:1333. https://doi.org/10.3389/fphys.2018.01333
Hu P, Zhou H, Lu M, Dou L, Bo G, Wu J, Huang S (2015) Autophagy plays a protective role in advanced glycation end product-induced apoptosis in cardiomyocytes. Cell Physiol Biochem 37:697–706. https://doi.org/10.1159/000430388
Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, He X, Wang N (2014) Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol 13:78. https://doi.org/10.1186/1475-2840-13-78
Li J, Wu PW, Zhou Y, Dai B, Zhang PF, Zhang YH, Liu Y, Shi XL (2018) Rage induces hepatocellular carcinoma proliferation and sorafenib resistance by modulating autophagy. Cell Death Dis 9:225. https://doi.org/10.1038/s41419-018-0329-z
Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, Cheng H, Jin K, Ni Q, Yu X (2019) The role of necroptosis in cancer biology and therapy. Mol Cancer 18:100
LaRocca TJ, Sosunov SA, Shakerley NL, Ten VS, Ratner AJ (2016) Hyperglycemic conditions prime cells for RIP1-dependent necroptosis. J Biol Chem 291:13753–13761
Su Z, Yang Z, Xu Y, Chen Y, Yu Q (2015) Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 14:48
Moreno-Gonzalez G, Vandenabeele P, Krysko DV (2016) Necroptosis: a novel cell death modality and its potential relevance for critical care medicine. Am J Respir Crit Care Med 194:415–428
Nogueira-Machado JA, Volpe CMDO, Veloso CA, Chaves MMJ (2011) HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert Opin Ther Targets 15:1023–1035
Wu L, Yang L (2018) The function and mechanism of HMGB1 in lung cancer and its potential therapeutic implications. Oncol Lett 15:6799–6805
Li X, Jiang S, Tapping RI (2010) Toll-like receptor signaling in cell proliferation and survival. Cytokine 49:1–9
Nogueira-Machado JA, Volpe CMDO, Veloso CA, Chaves MM (2011) HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Exp Opin Ther Targets 15:1023–1035
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Zhang C, Dong H, Chen F, Wang Y, Ma J, Wang G (2019) The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. Exp Ther Med 17:17–26
Alcalá S, Sainz B (2019) The dark side of radiotherapy-induced cell death in cancer. EBioMedicine 40:7–8
O'Neill LA, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7:353–364
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ (2009) HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367–388
Tang D, Kang R, Zeh HJ III, Lotze MT (2010) High-mobility group box 1 and cancer. Biochem Biophys Acta 1799:131–140
Zhang T, Guan X-W, Gribben JG, Liu F-T, Jia L (2019) Blockade of HMGB1 signaling pathway by ethyl pyruvate inhibits tumor growth in diffuse large B-cell lymphoma. Cell Death Dis 10:1–15
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Alvarez-Garcia I, Miska EA (2005) MicroRNA functions in animal development and human disease. Development 132:4653–4662
Zhang B, Pan X, Cobb GP, Anderson TA (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302:1–12
Bracken CP, Scott HS, Goodall GJ (2016) A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet 17:719–732
Choudhury Y, Tay FC, Lam DH, Sandanaraj E, Tang C, Ang B-T, Wang S (2012) Attenuated adenosine-to-inosine editing of microRNA-376a* promotes invasiveness of glioblastoma cells. J Clin Investig 122:4059–4076
Lin S, Gregory RI (2015) MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 15:321–333
Dvinge H, Git A, Gräf S, Salmon-Divon M, Curtis C, Sottoriva A, Zhao Y, Hirst M, Armisen J, Miska EA (2013) The shaping and functional consequences of the microRNA landscape in breast cancer. Nature 497:378–382
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA (2005) MicroRNA expression profiles classify human cancers. Nature 435:834–838
Mercado-Pimentel ME, Onyeagucha BC, Li Q, Pimentel AC, Jandova J, Nelson MA (2015) The S100P/RAGE signaling pathway regulates expression of microRNA-21 in colon cancer cells. FEBS Lett 589:2388–2393
Faltejskova P, Besse A, Sevcikova S, Kubiczkova L, Svoboda M, Smarda J, Kiss I, Vyzula R, Slaby O (2012) Clinical correlations of miR-21 expression in colorectal cancer patients and effects of its inhibition on DLD1 colon cancer cells. Int J Colorectal Dis 27:1401–1408
Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C (2009) A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 104:476–487
Mardente S, Mari E, Consorti F, Di Gioia C, Negri R, Etna M, Zicari A, Antonaci A (2012) HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells. Oncol Rep 28:2285–2289
Mardente S, Mari E, Massimi I, Fico F, Faggioni A, Pulcinelli F, Antonaci A, Zicari A (2015) HMGB1-induced cross talk between PTEN and miRs 221/222 in thyroid cancer. Biomed Res Int 2015:512027
Li L-M, Hou D-X, Guo Y-L, Yang J-W, Liu Y, Zhang C-Y, Zen K (2011) Role of microRNA-214–targeting phosphatase and tensin homolog in advanced glycation end product-induced apoptosis delay in monocytes. J Immunol 186:2552–2560
Qin Y, Ye J, Wang P, Gao L, Wang S, Shen H (2016) miR-223 contributes to the AGE-promoted apoptosis via down-regulating insulin-like growth factor 1 receptor in osteoblasts. Biosci Rep 36:e00314
Wang L, Kang F-B, Wang J, Yang C, He D-W (2019) Downregulation of miR-205 contributes to epithelial–mesenchymal transition and invasion in triple-negative breast cancer by targeting HMGB1–RAGE signaling pathway. Anticancer Drugs 30:225
El-Far AHAM, Munesue S, Harashima A, Sato A, Shindo M, Nakajima S, Inada M, Tanaka M, Takeuchi A, Tsuchiya H (2018) In vitro anticancer effects of a RAGE inhibitor discovered using a structure-based drug design system. Oncol Lett 15:4627–4634
Jimenez R, Lopez-Sepulveda R, Romero M, Toral M, Cogolludo A, Perez-Vizcaino F, Duarte J (2015) Quercetin and its metabolites inhibit the membrane NADPH oxidase activity in vascular smooth muscle cells from normotensive and spontaneously hypertensive rats. Food Funct 6:409–414
Hahm E-R, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV (2011) Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 6:e23354
Park JW, Min K-J, Kim DE, Kwon TK (2015) Withaferin A induces apoptosis through the generation of thiol oxidation in human head and neck cancer cells. Int J Mol Med 35:247–252
Arumugam T, Ramachandran V, Gomez SB, Schmidt AM, Logsdon CD (2012) S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin Cancer Res 18:4356–4364
Song T-Y, Yang N-C, Chen C-L, Thi TLV (2017) Protective effects and possible mechanisms of ergothioneine and hispidin against methylglyoxal-induced injuries in rat pheochromocytoma cells. Oxid Med Cell Longev. https://doi.org/10.1155/2017/4824371
Pellegrini L, Xue J, Larson D, Pastorino S, Jube S, Forest KH, Saad-Jube ZS, Napolitano A, Pagano I, Negi VS (2017) HMGB1 targeting by ethyl pyruvate suppresses malignant phenotype of human mesothelioma. Oncotarget 8:22649
Lee YR, Kim KM, Jeon BH, Choi S (2015) Extracellularly secreted APE1/Ref-1 triggers apoptosis in triple-negative breast cancer cells via RAGE binding, which is mediated through acetylation. Oncotarget 6:23383
Acknowledgements
SERB, Department of Science & Technology, New Delhi, Government of India is acknowledged for providing research Grant (EMR/2016/002574) to Chandramani Pathak. ICMR and DST-INSPIRE are gratefully acknowledged for providing senior research fellowship to Foram U. Vaidya and Bhargav N. Waghela. Indian Institute of Advanced Research, Puri foundation for education in India is gratefully acknowledged for providing research Infrastructure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waghela, B.N., Vaidya, F.U., Ranjan, K. et al. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol Cell Biochem 476, 585–598 (2021). https://doi.org/10.1007/s11010-020-03928-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03928-y