Abstract
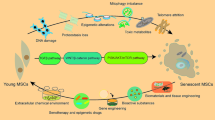
β-cell dysfunction is a critical determinant for both type 1 diabetes and type 2 diabetes and β-cells are shown to be highly susceptible to cellular stressors. Mesenchymal stem cells (MSCs) on the other hand are known to have immunomodulatory potential and preferred in clinical applications. However, there is paucity of a comparative study on these cells in relation to several cellular stressors in response to hyperglycemia and this forms the rationale for the present study. INS1 β-cells and MSCs were subjected to high-glucose treatment without and with Metformin, Lactoferrin, or TUDCA and assessed for stress signaling alterations using gene expression, protein expression, as well as functional read-outs. Compared to the untreated control cells, INS1 β-cells or MSCs treated with high glucose showed significant increase in mRNA expressions of ER stress, senescence, and proinflammation. This was accompanied by increased miR146a target genes and decreased levels of SIRT1, NRF2, and miR146a in both the cell types. Consistent with the mRNA results, protein expression levels do reflect the same alterations. Notably, the alterations are relatively less extent in MSCs compared to INS1 β-cells. Interestingly, three different agents, viz., Metformin, Lactoferrin, or TUDCA, were found to overcome the high glucose-induced cellular stresses in a concerted and inter-linked way and restored the proliferation and migration capacity in MSCs as well as normalized the glucose-stimulated insulin secretion in INS1 β-cells. While our study gives a directionality for potential supplementation of metformin/lactoferrin/TUDCA in optimization protocols of MSCs, we suggest that in vitro preconditioning of MSCs with such factors should be further explored with in-depth investigations to harness and enhance the therapeutic capacity/potential of MSCs.







Similar content being viewed by others
References
International Diabetes Federation (2019) IDF diabetes atlas, 9th edn. International Diabetes Federation. Brussels, Belgium, pp 1–176
Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V (2013) Type diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y AcadSci 1281:51–63. https://doi.org/10.1111/j.1749-6632.2012.06838.x
Mohan V, Amutha A, Ranjani H, Unnikrishnan R, Datta M, Anjana RM, Staimez L, Ali MK, Narayan KM (2013) Associations of β-cell function and insulin resistance with youth-onset type 2 diabetes and prediabetes among Asian Indians. Diabetes technology & therapeutics 15(4):315–322. https://doi.org/10.1089/dia.2012.0259
Staimez LR, Weber MB, Ranjani H, Ali MK, Echouffo-Tcheugui JB, Phillips LS, Mohan V, Narayan KM (2013) Evidence of reduced β-cell function in Asian Indians with mild dysglycemia. Diabetes Care 36(9):2772–2778. https://doi.org/10.2337/dc12-2290
Poitout V, Robertson RP (2008) Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 29(3):351–366. https://doi.org/10.1210/er.2007-0023
Dingreville F, Panthu B, Thivolet C, Ducreux S, Gouriou Y, Pesenti S, Chauvin MA, Chikh K, Errazuriz-Cerda E, Van Coppenolle F, Rieusset J, Madec AM (2019) Differential effect of glucose on er-mitochondria Ca(2+) exchange participates in insulin secretion and glucotoxicity-mediated dysfunction of β-cells. Diabetes 68(9):1778–1794. https://doi.org/10.2337/db18-1112
Bensellam M, Jonas JC, Laybutt DR (2018) Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol 236(2):R109–R143. https://doi.org/10.1530/JOE-17-0516
Lytrivi M, Castell AL, Poitout V, Cnop M (2020) Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J MolBiol 432(5):1514–1534. https://doi.org/10.1016/j.jmb.2019.09.016
Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, Chen FF, Jiang XD (2013) Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation 10:106. https://doi.org/10.1186/1742-2094-10-106
Motegi SI, Sekiguchi A, Uchiyama A, Uehara A, Fujiwara C, Yamazaki S, Perera B, Nakamura H, Ogino S, Yokoyama Y, Akai R, Iwawaki T, Ishikawa O (2017) Protective effect of mesenchymal stem cells on the pressure ulcer formation by the regulation of oxidative and endoplasmic reticulum stress. Sci Rep 7(1):17186. https://doi.org/10.1038/s41598-017-17630-5
He Y, Zhang D, Zeng Y, Ma J, Wang J, Guo H, Zhang J, Wang M, Zhang W, Gong N (2018) Bone marrow-derived mesenchymal stem cells protect islet grafts against endoplasmic reticulum stress-induced apoptosis during the early stage after transplantation. Stem Cells 36(7):1045–1061. https://doi.org/10.1002/stem.2823
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, Shen J, Cheng Y, Fu X, Han W (2012) Infusion of mesenchymal stem cells ameliorate hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes 61(6):1616–1625. https://doi.org/10.2337/db11-1141
Hao H, Liu J, Shen J, Zhao Y, Liu H, Hou Q, Tong C, Ti D, Dong L, Cheng Y, Mu Y, Liu J, Fu X, Han W (2013) Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats. BiochemBiophys Res Commun 436(3):418–423. https://doi.org/10.1016/j.bbrc.2013.05.117
Xie M, Hao HJ, Cheng Y, Xie ZY, Yin YQ, Zhang Q, Gao JQ, Liu HY, Mu YM, Han WD (2017) Adipose-derived mesenchymal stem cells ameliorate hyperglycemia through regulating hepatic glucose metabolism in type 2 diabetic rats. BiochemBiophys Res Commun 483(1):435–441. https://doi.org/10.1016/j.bbrc.2016.12.125
Guan LX, Guan H, Li HB, Ren CA, Liu L, Chu JJ, Dai LJ (2015) Therapeutic efficacy of umbilical cord-derived mesenchymal stem cells in patients with type 2 diabetes. ExpTher Med 9(5):1623–1630. https://doi.org/10.3892/etm.2015.2339
Schäfer R, Spohn G, Baer PC (2016) Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy? Transfus Med Hemother 43(4):256–267. https://doi.org/10.1159/000447458
Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J (2017) Senescence of mesenchymal stem cells. Int J Mol Med 39(4):775–782. https://doi.org/10.3892/ijmm.2017.2912
Gharibi B, Hughes FJ (2012) Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med 1:771–782. https://doi.org/10.5966/sctm.2010-0031
Balasubramanyam M, Adaikalakoteswari A, Sampathkumar R, Mohan V (2006) Oxidative stress in Asian Indians with type 2 diabetes. In: Mohan V, Rao GHR (eds) Type 2 diabetes in South Asians: epidemiology, risk factors and prevention. Jaypee Brothers, Medical Publishers (P) Ltd., New Delhi, pp 164–172
Gokulakrishnan K, Mohanavalli KT, Monickaraj F, Mohan V, Balasubramanyam M (2009) Subclinical inflammation/oxidation as revealed by altered gene expression profiles in subjects with impaired glucose tolerance and Type 2 diabetes patients. Mol Cell Biochem 324(1–2):173–181. https://doi.org/10.1007/s11010-008-9996-x
Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, Mohan V (2011) Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol Cell Biochem 351(1–2):197–205. https://doi.org/10.1007/s11010-011-0727-3
Monickaraj F, Aravind S, Gokulakrishnan K, Sathishkumar C, Prabu P, Prabu D, Mohan V, Balasubramanyam M (2012) Accelerated aging as evidenced by increased telomere shortening and mitochondrial DNA depletion in patients with type 2 diabetes. Mol Cell Biochem 365(1–2):343–350. https://doi.org/10.1007/s11010-012-1276-0
Lenin R, Sankaramoorthy A, Mohan V, Balasubramanyam M (2015) Altered immunometabolism at the interface of increased endoplasmic reticulum (ER) stress in patients with type 2 diabetes. J LeukocBiol 98(4):615–622. https://doi.org/10.1189/jlb.3A1214-609R
Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M (2018) Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Hum Genomics 12(1):41. https://doi.org/10.1186/s40246-018-0173-3
Adaikalakoteswari A, Balasubramanyam M, Mohan V (2005) Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabet Med 22(9):1151–1156. https://doi.org/10.1111/j.1464-5491.2005.01574.x
Gorasia DG, Dudek NL, Veith PD, Shankar R, Safavi-Hemami H, Williamson NA, Reynolds EC, Hubbard MJ, Purcell AW (2015) Pancreatic beta cells are highly susceptible to oxidative and ER stresses during the development of diabetes. J Proteome Res 14(2):688–699. https://doi.org/10.1021/pr500643h
Li N, Liu F, Yang P, Xiong F, Yu Q, Li J, Zhou Z, Zhang S, Wang CY (2019) Aging and stress induced β cell senescence and its implication in diabetes development. Aging (Albany NY) 11(21):9947–9959. https://doi.org/10.18632/aging.102432
Alipoor B, Ghaedi H, Meshkani R, Torkamandi S, Saffari S, Iranpour M, Omrani MD (2017) Association of MiR-146a expression and type 2 diabetes mellitus: a meta-analysis. Int J Mol Cell Med 6(3):156–163. https://doi.org/10.22088/acadpub
Mensà E, Giuliani A, Matacchione G, Gurău F, Bonfigli AR, Romagnoli F, De Luca M, Sabbatinelli J, Olivieri F (2019) Circulating miR-146a in healthy aging and type 2 diabetes: age- and gender-specific trajectories. Mech Ageing Dev 180:1–10. https://doi.org/10.1016/j.mad.2019.03.001
Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N, Fujimiya M (2020) Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep 10(1):10772. https://doi.org/10.1038/s41598-020-67460-1
Wan S, Wu Q, Ji Y, Fu X, Wang Y (2020) Promotion of the immunomodulatory properties and osteogenic differentiation of adipose-derived mesenchymal stem cells in vitro by lentivirus-mediated miR-146a sponge expression. J Tissue EngRegen Med. https://doi.org/10.1002/term.3113
Ge Q, Xie XX, Xiao X, Li X (2019) Exosome-like vesicles as new mediators and therapeutic targets for treating insulin resistance and β-cell mass failure in type 2 diabetes mellitus. J Diabetes Res 2019:3256060. https://doi.org/10.1155/2019/3256060
Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P (2011) Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res 317(11):1541–1547. https://doi.org/10.1016/j.yexcr.2011.02.015
Burova E, Borodkina A, Shatrova A, Nikolsky N (2013) Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxid Med Cell Longev 2013:474931. https://doi.org/10.1155/2013/474931
Alekseenko LL, Zemelko VI, Domnina AP, Lyublinskaya OG, Zenin VV, Pugovkina NA, Kozhukharova IV, Borodkina AV, Grinchuk TM, Fridlyanskaya II, Nikolsky NN (2014) Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell Stress Chaperones 19(3):355–366. https://doi.org/10.1007/s12192-013-0463-6
Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R (2010) Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev 19(12):1875–1884. https://doi.org/10.1089/scd.2010.0009
Alicka M, Major P, Wysocki M, Marycz K (2019) Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. J Clin Med 8(6):765. https://doi.org/10.3390/jcm8060765
Saparov A, Ogay V, Nurgozhin T, Jumabay M, Chen WC (2016) Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Intn 2016:3924858. https://doi.org/10.1155/2016/3924858
Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM (2018) Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol 9:2837. https://doi.org/10.3389/fimmu.2018.02837
Kouroupis D, Sanjurjo-Rodriguez C, Jones E, Correa D (2019) Mesenchymal stem cell functionalization for enhanced therapeutic applications. Tissue Eng Part B Rev 25(1):55–77. https://doi.org/10.1089/ten.TEB.2018.0118
Xiang E, Han B, Zhang Q, Rao W, Wang Z, Chang C, Zhang Y, Tu C, Li C, Wu D (2020) Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther. 11(1):336. https://doi.org/10.1186/s13287-020-01852-y
Alvaro-Afonso FJ, Sanz-Corbalan I, Lazaro-Martinez JL, Kakagia D, Papanas N (2020) Adipose-derived mesenchymal stem cells in the treatment of diabetic foot ulcers: a review of preclinical and clinical studies. Angiology 71(9):853–863. https://doi.org/10.1177/0003319720939467
He Q, Wang L, Zhao R, Yan F, Sha S, Cui C, Song J, Hu H, Guo X, Yang M, Cui Y, Sun Y, Sun Z, Liu F, Dong M, Hou X, Chen L (2020) Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Res Ther 11(1):223. https://doi.org/10.1186/s13287-020-01731-6
Kulkarni AS, Gubbi S, Barzilai N (2020) Benefits of metformin in attenuating the hallmarks of aging. Cell Metab 32(1):15–30. https://doi.org/10.1016/j.cmet.2020.04.001
Sabra S, Agwa MM (2020) Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int J BiolMacromol 164:1046–1060. https://doi.org/10.1016/j.ijbiomac.2020.07.167
Kusaczuk M (2019) Tauroursodeoxycholate-bile acid with chaperoning activity: molecular and cellular effects and therapeutic perspectives. Cells 8(12):1471. https://doi.org/10.3390/cells8121471
Hao L, Shan Q, Wei J, Ma F, Sun P (2019) Lactoferrin: major physiological functions and applications. Curr Protein PeptSci 20(2):139–144. https://doi.org/10.2174/1389203719666180514150921
Park SY, Jeong AJ, Kim GY, Jo A, Lee JE, Leem SH, Yoon JH, Ye SK, Chung JW (2017) Lactoferrin protects human mesenchymal stem cells from oxidative stress-induced senescence and Apoptosis. J MicrobiolBiotechnol 27(10):1877–1884. https://doi.org/10.4014/jmb.1707.07040
Xu Y, An JJ, Tabys D, Xie YD, Zhao TY, Ren HW, Liu N (2019) Effect of lactoferrin on the expression profiles of long non-coding RNA during osteogenic differentiation of bone marrow mesenchymal stem cells. Int J Mol Sci 20(19):4834. https://doi.org/10.3390/ijms20194834
Icriverzi M, Bonciu A, Rusen L, Sima LE, Brajnicov S, Cimpean A, Evans RW, Dinca V, Roseanu A (2019) Human mesenchymal stem cell response to lactoferrin-based composite coatings. Materials (Basel) 12(20):3414. https://doi.org/10.3390/ma12203414
Yoon YM, Kim S, Han YS, Yun CW, Lee JH, Noh H, Lee SH (2019) TUDCA-treated chronic kidney disease-derived hMSCs improve therapeutic efficacy in ischemic disease via PrP(C). Redox Biol 27(5):256-258. https://doi.org/10.1016/j.redox.2019.101144
Lee JH, Yoon YM, Lee SH (2019) TUDCA-treated mesenchymal stem cells protect against ER stress in the hippocampus of a murine chronic kidney disease model. Int J MolSci 20(3):613. https://doi.org/10.3390/ijms20030613
Balasubramanyam M (2014) Metformin–nature’s gift that keeps on giving more. J ObesMetab Res 1:118–120. https://doi.org/10.4103/2347-9906.134428
Zhou J, Massey S, Story D, Li L (2018) Metformin: an old drug with new applications. Int J Mol Sci 19(10):2863. https://doi.org/10.3390/ijms19102863
Park MJ, Moon SJ, Baek JA, Lee EJ, Jung KA, Kim EK, Kim DS, Lee JH, Kwok SK, Min JK, Kim SJ, Park SH, Cho ML (2019) Metformin augments anti-inflammatory and chondroprotective properties of mesenchymal stem cells in experimental osteoarthritis. J Immunol 203(1):127–136. https://doi.org/10.4049/jimmunol.1800006
Jang SG, Lee J, Hong SM, Kwok SK, Cho ML, Park SH (2020) Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology (Oxford) 59(6):1426-1438. https://doi.org/10.1093/rheumatology/kez631
Shree N, Bhonde RR (2016) Metformin preconditioned adipose derived mesenchymal stem cells is a better option for the reversal of diabetes upon transplantation. Biomed Pharmacother 84:1662–1667. https://doi.org/10.1016/j.biopha.2016.10.086
Shawky LM, El Bana EA, Morsi AA (2019) Stem cells and metformin synergistically promote healing in experimentally induced cutaneous wound injury in diabetic rats. Folia HistochemCytobiol 57(3):127–138. https://doi.org/10.5603/FHC.a2019.0014
Acknowledgements
The authors acknowledge partial grant support from the Indian Council of Medical Research (ICMR), Govt. of India. Srividhya Raghavan acknowledges the Council of Scientific & Industrial Research (CSIR), New Delhi, India for her financial assistance as Senior Research Fellowship. The work is part of the PhD topic of Srividhya Raghavan from the Madras Diabetes Research Foundation––which is affiliated to the University of Madras for PhD curriculum.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no actual or potential competing financial interests. They also declare that they do have any non-financial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raghavan, S., Malayaperumal, S., Mohan, V. et al. A comparative study on the cellular stressors in mesenchymal stem cells (MSCs) and pancreatic β-cells under hyperglycemic milieu. Mol Cell Biochem 476, 457–469 (2021). https://doi.org/10.1007/s11010-020-03922-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03922-4