Abstract
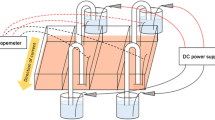
Human dermal fibroblast proliferation plays an important role in skin wound healing, and electrical stimulation (ES) promotes skin wound healing. Although the use of ES for skin wound healing has been investigated, the mechanism underlying the effects of ES on cells is still unclear. This study examined the effects of pulsed electrical stimulation (PES) on human dermal fibroblasts. Normal adult human dermal fibroblasts were exposed to a frequency of 4800 Hz, voltage of 1–5 V, and PES exposure time of 15, 30, and 60 min. Dermal fibroblast proliferation and growth factor gene expression were investigated for 6–48 h post PES. Dermal fibroblast proliferation significantly increased from 24 to 48 h post PES at a voltage of 5 V and PES exposure time of 60 min. Under the same conditions, post PES, platelet-derived growth factor subunit A (PDGFA), fibroblast growth factor 2 (FGF2), and transforming growth factor beta 1 (TGF-β1) expression significantly increased from 6 to 24 h, 12 to 48 h, and 24 to 48 h, respectively. Imatinib, a specific inhibitor of platelet-derived growth factor receptor, significantly inhibited the proliferation of dermal fibroblasts promoted by PES, suggesting that PDGFA expression, an early response of PES, was involved in promoting the cell proliferation. Therefore, PES at 4800 Hz may initially promote PDGFA expression and subsequently stimulate the expression of two other growth factors, resulting in dermal fibroblast proliferation after 24 h or later. In conclusion, PES may activate the cell growth phase of wound healing.




Similar content being viewed by others
References
Nuccitelli R, Nucciteli P, Li C, Narsing S, Pariser DM, Lui K (2011) The electric field near human skin wounds declines with age and provides a non-invasive indicator of wound healing. Wound Repair Regen 19(5):645–655
Zhao M (2008) Electrical fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol 20(6):674–682
Guo A, Song B, Reid B, Gu Y, Forrester JV, Jahoda CA, Zhao M (2010) Effects of physiological electric fields on migration of human dermal fibroblasts. J Investig Dermatol 130(9):2320–2327
Kim MS, Lee MH, Kwon BJ, Seo HJ, Koo MA, You KE, Kim D, Park JC (2015) Control of neonatal human dermal fibroblast migration on poly (lactic-co-glycolic acid)-coated surfaces by electrotaxis. J Tissue Eng Regen Med 11(3):862–868. https://doi.org/10.1002/term.1986
Kim MS, Lee MH, Kwon BJ, Koo MA, Seon GM, Park JC (2015) Golgi polarization plays a role in the directional migration of neonatal dermal fibroblasts induced by the direct current electric fields. Biochem Biophys Res Commun 460(2):255–260. https://doi.org/10.1016/j.bbrc.2015.03.021
Sarah S, Carlisle D, Rebecca KW (2017) Electrical stimulation increases random migration of human dermal fibroblasts. Ann Biomed Eng 45(9):2049–2060. https://doi.org/10.1007/s10439-017-1849-x
Gyu SL, Min GK, Hyuck JK (2019) Electrical stimulation induces direct reprogramming of human dermal fibroblasts into hyaline chondrogenic cells. Biochem Biophys Res Commun 513(4):990–996. https://doi.org/10.1016/j.bbrc.2019.04.027
Clark RA (1993) Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci 306(1):42–48
Yoshiyuki Y, Masaharu S, Mikiko U, Masafumi M (2016) Monophasic pulsed microcurrent of 1–8 Hz increases the number of human dermal fibroblasts. Prog Rehabil Med 1:20160005. https://doi.org/10.2490/prm.20160005
Suting L, Danhua L, Jianming T, Jie M, Ming H, Yang L, Yaodan L, Linlin W, Cheng L, Li H (2019) Electrical stimulation activates fibroblasts through the elevation of intracellular free Ca2+ potential mechanism of pelvic electrical stimulation therapy. Biomed Res Int 2019:7387803. https://doi.org/10.1155/2019/7387803 (eCollection 2019)
Rouabhia M, Park H, Meng S, Derbali H, Zhang Z (2013) (2013) Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS One 8(8):e71660. https://doi.org/10.1371/journal.pone.0071660 (eCollection 2013)
Wang Y, Rouabhia M, Lavertu D, Zhang Z (2014) Pulsed electrical stimulation modulates fibroblasts’ behaviour through the Smad signalling pathway. J Tissue Eng Regen Med 11(4):1110–1121. https://doi.org/10.1002/term.2014
Ojingwa JC, Isseroff RR (2003) Electrical stimulation of wound healing. J Investig Dermatol 121(1):1–12. https://doi.org/10.1046/j.1523-1747.2003.12454.x
Feedar JA, Kloth LC, Gentzkow GD (1991) Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther 71(9):639–649
Masashi M, Kousei T (1985) Physical therapy in practice. Nanzando, Tokyo
Tetsuo T (1979) Introduction to electrotherapy. Kenyukan, Tokyo
Hori Y, Akimoto R, Hori A, Kato K, Chino D, Matsumoto S, Kamiya S, Watanabe Y (2009) Skin collagen reproduction increased by ascorbic acid derivative iontophoresis by frequent-reversal bipolar electric stimulation. J Cosmet Sci 60(4):415–422
Arai KY, Nakamura Y, Hachiya Y, Tsuchiya H, Akimoto R, Hosoki K, Kamiya S, Ichikawa H, Nishiyama T (2013) Pulsed electric current induces the differentiation of human keratinocytes. Mol Cell Biochem 379(1–2):235–241. https://doi.org/10.1007/s11010-013-1645-3
Harrach S, Barz V, Pap T, Pavenstädt H (2019) Notch signaling activity determines uptake and biological effect of imatinib in systemic sclerosis dermal fibroblasts. J Investig Dermatol 139(2):439–447. https://doi.org/10.1016/j.jid.2018.08.021
Kameda H (2009) Imatinib. Jpn J Clin Immunol 32(2):77–84. https://doi.org/10.2177/jsci.32.77
Kim IS, Song JK, Zhang YL, Lee TH, Cho TH, Song YM, Kim DK, Kim SJ, Hwang SJ (2006) Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. Biochim Biophys Acta 1763(9):907–916
Borena BM, Martens A, Broeckx SY, Meyer E, Chiers K, Duchateau L, Spaas JH (2015) Regenerative skin wound healing in mammals: state-of-the-art on growth factor and stem cell based treatments. Cell Physiol Biochem 36(1):1–23. https://doi.org/10.1159/000374049
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870
Kiritsy CP, Lynch AB, Lynch SE (1993) Role of growth factors in cutaneous wound healing: a review. Crit Rev Oral Biol Med 4(5):729–760
Lynch SE, Nixon JC, Colvin RB, Antoniades HN (1987) Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci USA 84(21):7696–7700
Seppä H, Grotendorst G, Seppä S, Schiffmann E, Martin GR (1982) Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol 92(2):584–588
Shah JM, Omar E, Pai DR, Sood S (2012) Cellular events and biomarkers of wound healing. Indian J Plast Surg 45(2):220–228. https://doi.org/10.4103/0970-0358.101282
Tanaka R, Seki Y, Saito Y, Kamiya S, Fujita M, Okutsu H, Iyoda T, Takai T, Owaki T, Yajima H, Taira J, Hayashi R, Kodama H, Matsunaga T, Fukai F (2014) Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin α5β1. J Biol Chem 289(25):17699–17708. https://doi.org/10.1074/jbc.M113.546622
Horikawa S, Ishii Y, Hamashima T, Yamamoto S, Mori H, Fujimori T, Shen J, Inoue R, Nishizono H, Itoh H, Majima M, Abraham D, Miyawaki T, Sasahara M (2015) PDGFRα plays a crucial role in connective tissue remodeling. Sci Rep. https://doi.org/10.1038/srep17948
Boilly B, Vercoutter-Edouart AS, Hondermarck H, Nurcombe V, Le Bourhis X (2000) FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev 11(4):295–302
Makino T, Jinnin M, Muchemwa FC, Fukushima S, Kogushi-Nishi H, Moriya C, Igata T, Fujisawa A, Johno T, Ihn H (2010) Basic fibroblast growth factor stimulates the proliferation of human dermal fibroblasts via the ERK1/2 and JNK pathways. Br J Dermatol 162(4):717–723. https://doi.org/10.1111/j.1365-2133.2009.09581.x
Grazul-Bilska AT, Luthra G, Reynolds LP, Bilski JJ, Johnson ML, Adbullah SA, Redmer DA, Abdullah KM (2002) Effects of basic fibroblast growth factor (FGF-2) on proliferation of human skin fibroblasts in type II diabetes mellitus. Exp Clin Endocrinol Diabetes 110(4):176–181
Miyazono K (1996) Transforming growth factor-beta and its receptors. Nihon Yakurigaku Zasshi 107(3):133–140
Miyazono K, Ten Dijke P, Ichijo H, Heldin CH (1994) Receptors for transforming growth factor-beta. Adv Immunol 55:181–220
Cohen MH, Williams G, Johnson JR, Duan J, Gobburu J, Rahman A, Benson K, Leighton J, Kim SK, Wood R, Rothmann M, Chen G, KM U, Staten AM, Pazdur R (2002) Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res 8(5):935–942
Druker JB, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 2(5):561–566. https://doi.org/10.1038/nm0596-561
Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB (2000) Abl proteintyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet derived growth factor receptors. J Pharmacol Exp Ther 295(1):139–145
Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF (1989) Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol 109(1):429–440
Song QH, Klepeis VE, Nugent MA, Trinkaus-Randall V (2002) TGF-beta1 regulates TGF-beta1 and FGF-2 mRNA expression during fibroblast wound healing. Mol Pathol 55(3):164–176
Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, van Kooten C, Müller G (2001) TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int 59(2):579–592
Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K, Tammi MI (2011) Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J 78(9):1419–1428. https://doi.org/10.1111/j.1742-4658.2011.08070.x
Pasonen-Seppänen S, Karvinen S, Törrönen K, Hyttinen JM, Jokela T, Lammi MJ, Tammi MI, Tammi R (2003) EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J Investig Dermatol 120(6):1038–1044. https://doi.org/10.1046/j.1523-1747.2003.12249.x
Sugiyama Y, Shimada A, Sayo T, Sakai S, Inoue S (1998) Putative hyaluronan synthase mRNA are expressed in mouse skin and TGF-beta upregulates their expression in cultured human skin cells. J Investig Dermatol 110(2):116–121. https://doi.org/10.1046/j.1523-1747.1998.00093.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animal and human rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Urabe, H., Akimoto, R., Kamiya, S. et al. Effects of pulsed electrical stimulation on growth factor gene expression and proliferation in human dermal fibroblasts. Mol Cell Biochem 476, 361–368 (2021). https://doi.org/10.1007/s11010-020-03912-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03912-6