Abstract
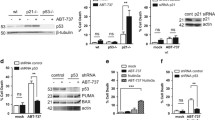
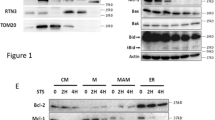
Ionizing radiation induces apoptosis in human Molt-4 leukemia cells in a p53-dependent manner. The tumor suppressor p53 stimulates various downstream targets that presumably trigger, individually or in concert, de novo ceramide synthesis and intrinsic apoptosis via mitochondrial outer membrane permeabilization (MOMP). Among these targets, BH3-only protein Noxa was found to be promptly activated by p53 prior to ceramide accumulation and apoptosis in response to irradiation. To evaluate the relation between Noxa and ceramide in irradiation-induced apoptosis, Noxa was silenced in Molt-4 cells and apoptosis, p53 expression, and ceramide accumulation were assessed in response to irradiation. In the absence of Noxa, irradiation of Molt-4 cells still induced apoptosis in a p53-dependent manner however ceramide levels decreased significantly although they remained higher than untreated control. Upon irradiation, Noxa was found to translocate to the mitochondria where endogenous ceramide accumulation was observed. In contrast, overexpression of Bcl-2, another mitochondrial protein, in Molt-4 cells abolished the endogenous ceramide accumulation and apoptosis. In irradiation-induced, p53-dependent pathways of apoptosis, the pro-apoptotic Noxa represents one of several, yet to be identified, pathways simultaneously triggered by p53 to produce mitochondrial ceramide accumulation and apoptosis. In contrast, Bcl-2 functions as a broader inhibitor of both ceramide accumulation and apoptosis. Altogether, these results indicate that members of the Bcl-2 family differentially regulate ceramide accumulation and reveal the existence of crosstalk between Bcl-2 family members and ceramide in mediating p53-dependent apoptosis in Molt-4 human T-cell leukemia.





Similar content being viewed by others
References
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Morad SA, Cabot MC (2013) Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13(1):51–65. https://doi.org/10.1038/nrc3398
Campaner S, Spreafico F, Burgold T, Doni M, Rosato U, Amati B, Testa G (2011) The methyltransferase Set7/9 (Setd7) is dispensable for the p53-mediated DNA damage response in vivo. Mol Cell 43(4):681–688. https://doi.org/10.1016/j.molcel.2011.08.007
Dbaibo GS, Pushkareva MY, Rachid RA, Alter N, Smyth MJ, Obeid LM, Hannun YA (1998) p53-dependent ceramide response to genotoxic stress. J Clin Invest 102(2):329–339. https://doi.org/10.1172/JCI1180
Sharpe JC, Arnoult D, Youle RJ (2004) Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta 1644(2–3):107–113. https://doi.org/10.1016/j.bbamcr.2003.10.016
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A (2003) p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302(5647):1036–1038. https://doi.org/10.1126/science.1090072
Leber B, Lin J, Andrews DW (2010) Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene 29(38):5221–5230. https://doi.org/10.1038/onc.2010.283
Lindsten T, Thompson CB (2006) Cell death in the absence of Bax and Bak. Cell Death Differ 13(8):1272–1276. https://doi.org/10.1038/sj.cdd.4401953
Shamas-Din A, Bindner S, Chi X, Leber B, Andrews DW, Fradin C (2015) Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax. Biochem J 467(3):495–505. https://doi.org/10.1042/BJ20141291
Vela L, Gonzalo O, Naval J, Marzo I (2013) Direct interaction of Bax and Bak proteins with Bcl-2 homology domain 3 (BH3)-only proteins in living cells revealed by fluorescence complementation. J Biol Chem 288(7):4935–4946. https://doi.org/10.1074/jbc.M112.422204
Colombini M (2017) Ceramide channels and mitochondrial outer membrane permeability. J Bioenerg Biomembr 49(1):57–64. https://doi.org/10.1007/s10863-016-9646-z
Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D (2004) Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J 382(Pt 2):527–533. https://doi.org/10.1042/BJ20031819
Perera MN, Lin SH, Peterson YK, Bielawska A, Szulc ZM, Bittman R, Colombini M (2012) Bax and Bcl-xL exert their regulation on different sites of the ceramide channel. Biochem J 445(1):81–91. https://doi.org/10.1042/BJ20112103
Panjarian S, Kozhaya L, Arayssi S, Yehia M, Bielawski J, Bielawska A, Usta J, Hannun YA, Obeid LM, Dbaibo GS (2008) De novo N-palmitoylsphingosine synthesis is the major biochemical mechanism of ceramide accumulation following p53 up-regulation. Prostaglandins Other Lipid Mediat 86(1–4):41–48. https://doi.org/10.1016/j.prostaglandins.2008.02.004
Hage-Sleiman R, Bahmad H, Kobeissy H, Dakdouk Z, Kobeissy F, Dbaibo G (2017) Genomic alterations during p53-dependent apoptosis induced by gamma-irradiation of Molt-4 leukemia cells. PLoS ONE 12(12):e0190221. https://doi.org/10.1371/journal.pone.0190221
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Rouser G, Fkeischer S, Yamamoto A (1970) Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5(5):494–496
Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM (1986) Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem 261(19):8597–8600
Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P (2009) Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc 4(11):1582–1590. https://doi.org/10.1038/nprot.2009.151
El-Assaad W, Kozhaya L, Araysi S, Panjarian S, Bitar FF, Baz E, El-Sabban ME, Dbaibo GS (2003) Ceramide and glutathione define two independently regulated pathways of cell death initiated by p53 in Molt-4 leukaemia cells. Biochem J 376(Pt 3):725–732. https://doi.org/10.1042/BJ20030888
Morsi RZ, Hage-Sleiman R, Kobeissy H, Dbaibo G (2018) Noxa: Role in Cancer Pathogenesis and Treatment. Curr Cancer Drug Targets 18(10):914–928. https://doi.org/10.2174/1568009618666180308105048
Ramirez-Camacho I, Bautista-Perez R, Correa F, Buelna-Chontal M, Roman-Anguiano NG, Medel-Franco M, Medina-Campos ON, Pedraza-Chaverri J, Cano-Martinez A (1862) Zazueta C (2016) Role of sphingomyelinase in mitochondrial ceramide accumulation during reperfusion. Biochim Biophys Acta 10:1955–1963. https://doi.org/10.1016/j.bbadis.2016.07.021
Law BA, Liao X, Moore KS, Southard A, Roddy P, Ji R, Szulc Z, Bielawska A, Schulze PC, Cowart LA (2018) Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J 32(3):1403–1416. https://doi.org/10.1096/fj.201700300R
Siskind LJ, Kolesnick RN, Colombini M (2002) Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem 277(30):26796–26803. https://doi.org/10.1074/jbc.M200754200
Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288(5468):1053–1058
Hoeferlin LA, Fekry B, Ogretmen B, Krupenko SA, Krupenko NI (2013) Folate stress induces apoptosis via p53-dependent de novo ceramide synthesis and up-regulation of ceramide synthase 6. J Biol Chem 288(18):12880–12890. https://doi.org/10.1074/jbc.M113.461798
Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, Nishimura Y, Sakai N, Nozawa Y (2000) Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene 19(31):3508–3520. https://doi.org/10.1038/sj.onc.1203699
Ploner C, Kofler R, Villunger A (2008) Noxa: at the tip of the balance between life and death. Oncogene 27(Suppl 1):S84–92. https://doi.org/10.1038/onc.2009.46
Huang B, Vassilev LT (2009) Reduced transcriptional activity in the p53 pathway of senescent cells revealed by the MDM2 antagonist nutlin-3. Aging (Albany NY) 1(10):845–854. https://doi.org/10.18632/aging.100091
Laviad EL, Kelly S, Merrill AH Jr, Futerman AH (2012) Modulation of ceramide synthase activity via dimerization. J Biol Chem 287(25):21025–21033. https://doi.org/10.1074/jbc.M112.363580
Jain A, Beutel O, Ebell K, Korneev S, Holthuis JC (2017) Diverting CERT-mediated ceramide transport to mitochondria triggers Bax-dependent apoptosis. J Cell Sci 130(2):360–371. https://doi.org/10.1242/jcs.194191
Bezombes C, Maestre N, Laurent G, Levade T, Bettaieb A, Jaffrezou JP (1998) Restoration of TNF-alpha-induced ceramide generation and apoptosis in resistant human leukemia KG1a cells by the P-glycoprotein blocker PSC833. FASEB J 12(1):101–109
Dbaibo GS, El-Assaad W, Krikorian A, Liu B, Diab K, Idriss NZ, El-Sabban M, Driscoll TA, Perry DK, Hannun YA (2001) Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death. FEBS Lett 503(1):7–12. https://doi.org/10.1016/s0014-5793(01)02625-4
Seo YW, Shin JN, Ko KH, Cha JH, Park JY, Lee BR, Yun CW, Kim YM, Seol DW, Kim DW, Yin XM, Kim TH (2003) The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J Biol Chem 278(48):48292–48299. https://doi.org/10.1074/jbc.M308785200
Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH (2011) Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J Cell Biol 194(1):39–48. https://doi.org/10.1083/jcb.201102027
Kim JY, An YM, Choi WH, Kim JM, Cho S, Yoo BR, Kang JW, Lee YS, Lee YJ, Cho J (2017) Pro-apoptotic Noxa is involved in ablative focal irradiation-induced lung injury. J Cell Mol Med 21(4):711–719. https://doi.org/10.1111/jcmm.13014
Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC (1993) Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res 53(19):4701–4714
Janiak F, Leber B, Andrews DW (1994) Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J Biol Chem 269(13):9842–9849
Thomenius MJ, Wang NS, Reineks EZ, Wang Z, Distelhorst CW (2003) Bcl-2 on the endoplasmic reticulum regulates Bax activity by binding to BH3-only proteins. J Biol Chem 278(8):6243–6250. https://doi.org/10.1074/jbc.M208878200
Smith AJ, Dai H, Correia C, Takahashi R, Lee SH, Schmitz I, Kaufmann SH (2011) Noxa/Bcl-2 protein interactions contribute to bortezomib resistance in human lymphoid cells. J Biol Chem 286(20):17682–17692. https://doi.org/10.1074/jbc.M110.189092
Allouche M, Bettaieb A, Vindis C, Rousse A, Grignon C, Laurent G (1997) Influence of Bcl-2 overexpression on the ceramide pathway in daunorubicin-induced apoptosis of leukemic cells. Oncogene 14(15):1837–1845. https://doi.org/10.1038/sj.onc.1201023
Dbaibo GS, Perry DK, Gamard CJ, Platt R, Poirier GG, Obeid LM, Hannun YA (1997) Cytokine response modifier A (CrmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-alpha: CrmA and Bcl-2 target distinct components in the apoptotic pathway. J Exp Med 185(3):481–490. https://doi.org/10.1084/jem.185.3.481
Funding
This research was supported by funding from the Medical Practice Plan (MPP) at AUB-FM and the Lebanese National Center for Scientific Research (LNCSR) to GD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
RHS and GD conceived and designed the project. HK, ZD and LK performed the experiments. HK and RHS analyzed the results. HK and RHS drafted the manuscript. RHS and GD revised and finalized the manuscript which was read and approved by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobeissy, H., Hage-Sleiman, R., Dakdouk, Z. et al. Crosstalk between Noxa, Bcl-2, and ceramide in mediating p53-dependent apoptosis in Molt-4 human T-cell leukemia. Mol Cell Biochem 475, 215–226 (2020). https://doi.org/10.1007/s11010-020-03874-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03874-9