Abstract
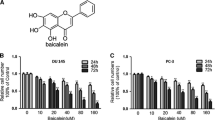
Chelerythrine is a natural benzo[c]phenanthridine alkaloid found in many herbs and displays a wide range of antitumor activities. Here, the present study tested their effects on prostate cancer cells. The addition of chelerythrine can significantly inhibit the proliferation of androgen-independent prostate cancer DU145 and PC-3 cells at the concentration of 5 and 10 μM, but not on androgen-dependent prostate cancer LNCaP cells as well as normal prostate epithelial cell line PrEC cells. Wound migration and transwell invasion assay showed the similar inhibitory effect of chelerythrine on the migration and invasion of DU145 and PC-3 cells in the same condition. Western blot analysis further confirmed that chelerythrine not only dramatically decreased MMP-2, MMP-9, and uPA protein expression, but also augmented the expression of their endogenous inhibitors (TIMP-1 and TIMP-2) and plasminogen activator inhibitors (PAI-1 and PAI-2) in both cancer cells. Meanwhile, NF-κB and AP-1 transcription factors were all suppressed as evidenced by the decline of p-p65, c-Fos, and c-Jun protein expression in both cells. Taken together, these findings suggested that chelerythrine could reduce the metastasis of androgen-independent prostate cancer cells via modulation of MMP/TIMP system and inactivation of NF-κB pathway.






Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- FBS:
-
Fetal bovine serum
- PrEC:
-
Prostate epithelial cells cell line
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamidegel electrophoresis
- SD:
-
Standard deviation
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
De Wang X, Su GY, Zhao C, Qu FZ, Wang P, Zhao YQ (2018) Anticancer activity and potential mechanisms of 1C, a ginseng saponin derivative, on prostate cancer cells. J Ginseng Res 42:133–143. https://doi.org/10.1016/j.jgr.2016.12.014
Mirza MB, Elkady AI, Al-Attar AM, Syed FQ, Mohammed FA, Hakeem KR (2018) Induction of apoptosis and cell cycle arrest by ethyl acetate fraction of Phoenix dactylifera L. (Ajwa dates) in prostate cancer cells. J Ethnopharmacol 218:35–44. https://doi.org/10.1016/j.jep.2018.02.030
Beltran H, Jendrisak A, Landers M, Mosquera JM, Kossai M, Louw J et al (2016) The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin Cancer Res 22:1510–1519. https://doi.org/10.1158/1078-0432.CCR-15-0137
Koczurkiewicz P, Kowolik E, Podolak I, Wnuk D, Piska K, Łabędź-Masłowska A et al (2016) Synergistic cytotoxic and anti-invasive effects of mitoxantrone and triterpene saponins from Lysimachia ciliata on human prostate cancer cells. Planta Med 82:1546–1552. https://doi.org/10.1055/s-0042-117537
Huang SH, Tseng JC, Lin CY, Kuo YY, Wang BJ, Kao YH et al (2019) Rooibos suppresses proliferation of castration-resistant prostate cancer cells via inhibition of Akt signaling. Phytomedicine 64:153068. https://doi.org/10.1016/j.phymed.2019.153068
Adsul P, Wray R, Spradling K, Darwish O, Weaver N, Siddiqui S (2015) Systematic review of decision aids for newly diagnosed prostate cancer patients making treatment decisions. J Urol 194:1247–1252. https://doi.org/10.1016/j.juro.2015.05.093
Tammela TL (2012) Endocrine prevention and treatment of prostate cancer. Mol Cell Endocrinol 360:59–67. https://doi.org/10.1016/j.mce.2012.03.002
Ryan CJ, Tindall DJ (2011) Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol 29:3651–3658. https://doi.org/10.1200/JCO.2011.35.2005
Huang MY, Zhang LL, Ding J, Lu JJ (2018) Anticancer drug discovery from Chinese medicinal herbs. Chin Med 13:35. https://doi.org/10.1186/s13020-018-0192-y
Malikova J, Zdarilova A, Hlobilkova A (2006) Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150:5–12. https://doi.org/10.5507/bp.2006.001
Zheng W, Qiu L, Wang R, Feng X, Han Y, Zhu Y et al (2015) Selective targeting of PPARγ by the natural product chelerythrine with a unique binding mode and improved antidiabetic potency. Sci Rep. https://doi.org/10.1038/srep12222
Yang XJ, Miao F, Yao Y, Cao FJ, Yang R, Ma YN et al (2012) In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules 17:13026–13035. https://doi.org/10.3390/molecules171113026
Vrba J, Doležel P, Vičar J, Modrianský M, Ulrichová J (2008) Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells. Toxicol In Vitro 22:1008–1017. https://doi.org/10.1016/j.tiv.2008.02.007
Tang ZH, Cao WX, Wang ZY, Lu JH, Liu B, Chen X, Lu JJ (2017) Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells. Redox Biol 12:367–376. https://doi.org/10.1016/j.redox.2017.03.009
Wu S, Yang Y, Li F, Huang L, Han Z, Wang G et al (2018) Chelerythrine induced cell death through ROS-dependent ER stress in human prostate cancer cells. Onco Targets Ther 11:2593. https://doi.org/10.2147/OTT.S157707
Wu B, Cui J, Zhang C, Li Z (2012) A polysaccharide from Agaricus blazei inhibits proliferation and promotes apoptosis of osteosarcoma cells. Int J Biol Macromol 50:1116–1120. https://doi.org/10.1016/j.ijbiomac.2012.02.023
Eisenbrand G, Pool-Zobel B, Baker V, Balls M, Blaauboer B, Boobis A et al (2002) Methods of in vitro toxicology. Food Chem Toxicol 40:193–236. https://doi.org/10.1016/S0278-6915(01)00118-1
Heo JC, Son M, Woo SU, Kweon M, Yoon EK, Lee HK et al (2008) A fraction of methylene chloride from Geum japonicum Thunberg inhibits tumor metastatic and angiogenic potential. Oncol Rep 19:1399–1403
Zhang D, Liu H, Yang B, Hu J, Cheng Y (2019) L-securinine inhibits cell growth and metastasis of human androgen-independent prostate cancer DU145 cells via regulating mitochondrial and AGTR1/MEK/ERK/STAT3/PAX2 apoptotic pathways. Biosci Rep. https://doi.org/10.1042/BSR20190469
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Manu KA, Kuttan G (2009) Anti-metastatic potential of Punarnavine, an alkaloid from Boerhaavia diffusa Linn. Immunobiology 214:245–255. https://doi.org/10.1016/j.imbio.2008.10.002
Park TY, Park MH, Shin WC, Rhee MH, Seo DW, Cho JY et al (2008) Anti-metastatic potential of ginsenoside Rp1, a novel ginsenoside derivative. Biol Pharm Bull 31:1802–1805. https://doi.org/10.1248/bpb.31.1802
Zell JA, Ou SH, Ziogas A, Anton-Culver H (2008) Cancer 112:136–143. https://doi.org/10.1002/cncr.23146
Wu HJ, Hao M, Yeo SK, Guan JL (2020) FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 39:2539–2549. https://doi.org/10.1038/s41388-020-1162-2
Yilmaz M, Christofori G, Lehembre F (2007) Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med 13:535–541. https://doi.org/10.1016/j.molmed.2007.10.004
Hu XX, He PP, Qi GB, Gao YJ, Lin YX, Yang C et al (2017) Transformable nanomaterials as an artificial extracellular matrix for inhibiting tumor invasion and metastasis. ACS Nano 11:4086–4096. https://doi.org/10.1021/acsnano.7b00781
Deng JS, Chang JS, Liao JC, Chao W, Lee MM, Cheng CH et al (2018) Actinidia callosa var. callosa suppresses metastatic potential of human hepatoma cell SK-Hep1 by inhibiting matrix metalloproteinase-2 through PI3K/Akt and MAPK signaling pathways. Bot Stud 59:1–11. https://doi.org/10.1186/s40529-017-0216-4
Sarwar MS, Zhang HJ, Tsang SW (2018) Perspectives of plant natural products in inhibition of cancer invasion and metastasis by regulating multiple signaling pathways. Curr Med Chem 25:5057–5087. https://doi.org/10.2174/0929867324666170918123413
Shan YF, Shen X, Xie YK, Chen JC, Shi HQ, Yu ZP et al (2009) Inhibitory effects of tanshinone II-A on invasion and metastasis of human colon carcinoma cells. Acta Pharmacol Sin 30:1537–1542. https://doi.org/10.1038/aps.2009.139
Hsu YL, Kuo PL, Cho CY, Ni WC, Tzeng TF, Ng LT et al (2007) Antrodia cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor κB pathway. Food Chem Toxicol 45:1249–1257. https://doi.org/10.1016/j.fct.2007.01.005
Bahassi EM, Karyala S, Tomlinson CR, Sartor MA, Medvedovic M, Hennigan RF (2004) Critical regulation of genes for tumor cell migration by AP-1. Clin Exp Metastasis 21:293–304. https://doi.org/10.1023/b:clin.0000046132.46946.dd
Huang SF, Chu SC, Hsieh YH, Chen PN, Hsieh YS (2018) Viola yedoensis suppresses cell invasion by targeting the protease and NF-κB activities in A549 and Lewis lung carcinoma cells. Int J Med Sci 15:280. https://doi.org/10.7150/ijms.22793
Baldwin AS (2001) Series introduction: the transcription factor NF-κB and human disease. J Clin Invest 107:3–6. https://doi.org/10.1172/JCI11891
Ho ML, Chen PN, Chu SC, Kuo DY, Kuo WH, Chen JY et al (2010) Peonidin 3-glucoside inhibits lung cancer metastasis by downregulation of proteinases activities and MAPK pathway. Nutr Cancer 62:505–516. https://doi.org/10.1080/01635580903441261
Funding
This study was supported by the Traditional Chinese Medicine Appropriate Technology Cultivation Project of Zhejiang Province (No: 2018ZT005) and Medical, Health Science and Technology Project of Zhejiang Province (No: 2019KY161).
Author information
Authors and Affiliations
Contributions
BY and DZ conceived and designed the study. BY, DZ, JQ, and YC performed the experiments. JQ, and YC analyzed the data. BY and DZ wrote and reviewed the final manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests associated with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, B., Zhang, D., Qian, J. et al. Chelerythrine suppresses proliferation and metastasis of human prostate cancer cells via modulating MMP/TIMP/NF-κB system. Mol Cell Biochem 474, 199–208 (2020). https://doi.org/10.1007/s11010-020-03845-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03845-0