Abstract
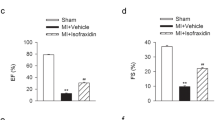
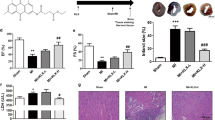
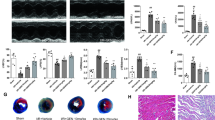
Artemisinin is known for its pharmaceutical effect against malaria and received increased attention for its other potential function. Mounting evidence suggest that artemisinin could also exert cardioprotective effects while the understanding of its regulatory mechanism is still limited. This study is designed to investigate the role of artemisinin in myocardial ischemia/reperfusion (I/R) injury and the involvement of NLRP3 inflammasome. Artemisinin was administrated for 14 consecutive days intragastrically before I/R injury. Cardiac function was assessed by echocardiography. Infarct area was observed through HE and TTC staining. Apoptosis and autophagy were assessed by TUNEL and Western blotting. The artemisinin-treated myocardial I/R rats demonstrated less severe myocardial I/R injury (smaller infarct size and lower CK-MB, LDH), significant inhibition of cardiac autophagy (decreased LC3II/I and increased p62), improved mitochondrial electron transport chain activity, concomitant with decreased activation of NLRP3 inflammasome (decreased NLRP3, ASC, cleaved caspase-1, IL-1β). In conclusion, our findings further confirmed that activation of the NLRP3 inflammasome pathway is involved in myocardial I/R injury, whereas artemisinin preconditioning could effectively protect against myocardial I/R injury through suppression of NLRP3 inflammasome activation. Therefore, the NLRP3 inflammasome might serve as a promising therapeutic target providing new mechanisms for understanding the effect of artemisinin during the evolution of myocardial infarction.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Yasuda S, Shimokawa H (2009) Acute myocardial infarction: the enduring challenge for cardiac protection and survival. Circ J 73:2000–2008
Weintraub WS, Sadanandan S (2003) Percutaneous coronary intervention in stable patients after acute myocardial infarction. Circulation 108:1292–1294
Dhaliwal H, Kirshenbaum LA, Randhawa AK, Singal PK (1991) Correlation between antioxidant changes during hypoxia and recovery on reoxygenation. Am J Physiol 261:632–638
Bing HW, Liew D, Huang KW, Li H, Tang W, Kelly DJ, Reid C, Liu Z (2018) The challenges of stem cell therapy in myocardial infarction and heart failure and the potential strategies to improve the outcomes. Nano Life 8:1841008
Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH (2001) Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol 280:2313–2320
Aghaei M, Motallebnezhad M, Ghorghanlu S, Jabbari A, Enayati A, Rajaei M, Pourabouk M, Moradi A, Alizadeh AM, Khori V (2019) Targeting autophagy in cardiac ischemia/reperfusion injury: a novel therapeutic strategy. J Cell Physiol 234:16768–16778
Jiang CM, Han LP, Li HZ, Qu YB, Zhang ZR, Wang R, Xu CQ, Li WM (2008) Calcium-sensing receptors induce apoptosis in cultured neonatal rat ventricular cardiomyocytes during simulated ischemia/reperfusion. Cell Biol Int 32:792–800
Zeng M, Wei X, Wu Z, Li W, Li B, Zhen Y, Chen J, Wang P, Fei Y (2013) NF-κB-mediated induction of autophagy in cardiac ischemia/reperfusion injury. Biochem Biophys Res Commun 436:180–185
Lutz J, Thürmel K, Heemann U (2010) Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. J Inflamm 7:27
Yang J, Li Y, Hu C (2011) Ischemic preconditioning protects against myocardial ischemia–reperfusion injury through inhibiting toll-like receptor 4/NF-κB signaling pathway in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36:972–978
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, Macdonald K, Speert D, Fernandesalnemri T, Wu J, Monks BG, Fitzgerald KA (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791
Daniels MJ, Rivers-Auty J, Schilling T, Spencer NG, Watremez W, Fasolino V, Booth SJ, White CS, Baldwin AG, Freeman S (2016) Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models. Nat Commun 7:12504
Menu P, Pellegrin M, Aubert J-F, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J (2011) Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis 2:e137
Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, Duan J (2017) The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed Pharmacother 91:1042–1052
He W, Long T, Pan Q, Zhang S, Zhang Y, Zhang D, Qin G, Chen L, Zhou J (2019) Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J Neuroinflamm 16:1–7
Liu X, Cao J, Huang G, Zhao Q, Shen J (2019) Biological activities of artemisinin derivatives beyond malaria. Curr Top Med Chem 19:205–222
Yao L, He JH, Xie H, Hu WL, Chen LJ (2009) Analyzing mechanism of artemisinin and its derivatives in anti-tumor by gene chip. China J Tradit Chin Med Pharm 24:1586–1589
Lu M, Sun L, Zhou J, Yang J (2014) Dihydroartemisinin induces apoptosis in colorectal cancer cells through the mitochondria-dependent pathway. Tumour Biol 35:5307–5314
Lu M, Sun L, Zhou J, Zhao Y, Deng X (2015) Dihydroartemisinin-induced apoptosis is associated with inhibition of sarco/endoplasmic reticulum calcium ATPase activity in colorectal cancer. Cell Biochem Biophys 73:137–145
Blazquez AG, Fernandezdolon M, Sanchezvicente L, Maestre AD, GomezSan Miguel AB, Alvarez M, Serrano MA, Jansen H, Efferth T, Marin JJ (2013) Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg Med Chem 21:4432–4441
Tan W, Feng S, Luo X, Su C, Qiu Z, Zeng H, Yan P, Yong Y, Wu M, Jiang X (2011) Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine 18:158–162
Gu Y, Wang X, Wang X, Yuan M, Wu G, Hu J, Tang Y, Huang C (2012) Artemisinin attenuates post-infarct myocardial remodeling by down-regulating the NF-κB pathway. Tohoku J Exp Med 227:161–170
Farombi EO, Adedara IA, Abolaji AO, Anamelechi JP, Sangodele JO (2014) Sperm characteristics, antioxidant status and hormonal profile in rats treated with artemisinin. Andrologia 46:893–901
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. Dig World Core Med J 46:608–608
Shi B, Ma M, Zheng Y, Pan Y, Lin X (2019) mTOR and Beclin1: two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J Cell Physiol 234:12562–12568
Gu Y, Wang X, Wu G, Wang X, Cao H, Tang Y, Huang C (2012) Artemisinin suppresses sympathetic hyperinnervation following myocardial infarction via anti-inflammatory effects. J Mol Histol 43:737–743
Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmidburgk J, Cavlar T, Hornung V (2011) Inflammasomes: current understanding and open questions. Cell Mol Life Sci 68:765–783
Martinon F, Gaide O, Pétrilli V, Mayor A, Tschopp J (2007) NALP inflammasomes central role in innate immunity. Semin Immunopathol 29:213
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464:1357–1361
Masanori K, Masafumi T, Takeki H, Yuichiro K, Fumitake U, Hajime M, Atsushi I, Yasuko T, Junya M, Jun K (2011) Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123:594–604
Øystein S, Trine R, LeifErik V, Marte BE, Katrine A, Finsen AV, Dahl CP, Askevold ET, Geir F, Geir C (2013) The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia–reperfusion injury. Cardiovasc Res 99:164–174
Long H, Xu B, Luo Y, Luo K (2016) Artemisinin protects mice against burn sepsis through inhibiting NLRP3 inflammasome activation. Am J Emerg Med 34:772–777
Lawlor KE, Vince JE (1840) Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? BBA 2014:1433–1440
Rongbin Z, Yazdi AS, Philippe M, Jürg T (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221
Latz E (2010) The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 22:28–33
Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L (2013) TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun 4:1611
Funding
This work was supported by The Fundamental Research Funds for the Provincial Universities (Grant No. 2017LCZX24) and the Foundation of the First Affiliated Hospital of Harbin Medical University (Grant No. 2018B017).
Author information
Authors and Affiliations
Contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Harbin Medical University and followed the National Institutes of Health (NIH) guidelines for laboratory animals care and use (NIH Pub. No. 85-23, revised 1996).
Informed consent
All authors have read and approved the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, F., Gao, Q., Yang, J. et al. Artemisinin suppresses myocardial ischemia–reperfusion injury via NLRP3 inflammasome mechanism. Mol Cell Biochem 474, 171–180 (2020). https://doi.org/10.1007/s11010-020-03842-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03842-3