Abstract
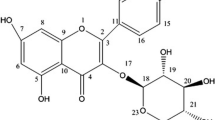
Plants are major source for discovery and development of anticancer drugs. Several plant-based anticancer drugs are currently in clinical use. Fagonia indica is a plant of medicinal value in the South Asian countries. Using mass spectrometry and NMR spectroscopy, several compounds were purified from the F. indica extract. We have used one of the purified compounds quinovic acid (QA) and found that QA strongly suppressed the growth and viability of human breast and lung cancer cells. QA did not inhibit growth and viability of non-tumorigenic breast cells. QA mediated its anticancer effects by inducing cell death. QA-induced cell death was associated with biochemical features of apoptosis such as activation of caspases 3 and 8 as well as PARP cleavage. QA also upregulated mRNA and protein levels of death receptor 5 (DR5). Further investigation revealed that QA did not alter DR5 gene promoter activity, but enhanced DR5 mRNA and protein stabilities. DR5 is one of the major components of the extrinsic pathway of apoptosis. Accordingly, Apo2L/TRAIL, the DR5 ligand, potentiated the anticancer effects of QA. Our results indicate that QA mediates its anticancer effects, at least in part, by engaging DR5-depentent pathway to induce apoptosis. Based on our results, we propose that QA in combination with Apo2L/TRAIL can be further investigated as a novel therapeutic approach for breast and lung cancers.







Similar content being viewed by others
References
Lichota A, Gwozdzinski K (2018) Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci 19:3533. https://doi.org/10.3390/ijms19113533
Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R (2009) Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci 5:1–11
Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109:3012–3043. https://doi.org/10.1021/cr900019j
Cragg GM, Newman DJ (2004) A tale of two tumor targets: topoisomerase I and tubulin. The Wall and Wani contribution to cancer chemotherapy. J Nat Prod 67:232–244. https://doi.org/10.1021/np030420c
Lau W, Sattely ES (2015) Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 349:1224–1228. https://doi.org/10.1126/science.aac7202
Shaker KH, Bernhardt M, Elgamal MH, Seifert K (1999) Triterpenoid saponins from Fagonia indica. Phytochemistry 51:1049–1053. https://doi.org/10.1016/s0031-9422(98)00750-x
Ullah N, Nadhman A, Siddiq S, Mehwish S, Islam A, Jafri L, Hamayun M (2016) Plants as antileishmanial agents: current scenario. Phytother Res 30:1905–1925. https://doi.org/10.1002/ptr.5710
Lam M, Carmichael AR, Griffiths HR (2012) An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS ONE 7:e40152. https://doi.org/10.1371/journal.pone.0040152
Waheed A, Barker J, Barton SJ, Owen CP, Ahmed S, Carew MA (2012) A novel steroidal saponin glycoside from Fagonia indica induces cell-selective apoptosis or necrosis in cancer cells. Eur J Pharm Sci 47:464–473. https://doi.org/10.1016/j.ejps.2012.07.004
Jafri L, Saleem S, Calderwood D, Gillespie A, Mirza B, Green BD (2016) Naturally-occurring TGR5 agonists modulating glucagon-like peptide-1 biosynthesis and secretion. Peptides 78:51–58. https://doi.org/10.1016/j.peptides.2016.01.015
Saleem S, Jafri L, ul Haq I, Chang LC, Calderwood D, Green BD, Mirza B (2014) Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. J Ethnopharmacol 156:26–32. https://doi.org/10.1016/j.jep.2014.08.017
Ahmed I, Islam M, Arshad W, Mannan A, Ahmad W, Mirza B (2009) High-quality plant DNA extraction for PCR: an easy approach. J Appl Genet 50:105–107. https://doi.org/10.1007/BF03195661
Group CPW (2009) A DNA barcode for land plants. Proc Natl Acad Sci USA 106:12794–12797. https://doi.org/10.1073/pnas.0905845106
Gjoerup O (2018) Growth curve (crystal violet staining and quantification protocol). https://labs.mmg.pitt.edu/gjoerup/growth%20curve%20cryst%20violet.doc. Accessed 15 Dec 2018
An J, Shi J, He Q, Lui K, Liu Y, Huang Y, Sheikh MS (2012) CHCM1/CHCHD6, novel mitochondrial protein linked to regulation of mitofilin and mitochondrial cristae morphology. J Biol Chem 287:7411–7426. https://doi.org/10.1074/jbc.M111.277103
Luo X, Huang Y, Sheikh MS (2003) Cloning and characterization of a novel gene PDRG that is differentially regulated by p53 and ultraviolet radiation. Oncogene 22:7247–7257. https://doi.org/10.1038/sj.onc.1207010
Shi J, He Q, An J, Sun H, Huang Y, Sheikh MS (2009) Sulindac sulfide differentially induces apoptosis in Smac-proficient and -deficient human colon cancer cells. Mol Cell Pharmacol 1:92–97. https://doi.org/10.4255/mcpharmacol.09.11
Takimoto R, El-Deiry WS (2000) Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene 19:1735–1743. https://doi.org/10.1038/sj.onc.1203489
Lui K, An J, Montalbano J, Shi J, Corcoran C, He Q, Sun H, Sheikh MS, Huang Y (2013) Negative regulation of p53 by Ras superfamily protein RBEL1A. J Cell Sci 126:2436–2445. https://doi.org/10.1242/jcs.118117
Fox J (2005) Getting started with the R commander: a basic-statistics graphical user interface to R. J Stat Softw 14:1–42
He Q, Lee DI, Rong R, Yu M, Luo X, Klein M, El-Deiry WS, Huang Y, Hussain A, Sheikh MS (2002) Endoplasmic reticulum calcium pool depletion-induced apoptosis is coupled with activation of the death receptor 5 pathway. Oncogene 21:2623–2633. https://doi.org/10.1038/sj.onc.1205345
Huang Y, Sheikh MS (2007) TRAIL death receptors and cancer therapeutics. Toxicol Appl Pharmacol 224:284–289. https://doi.org/10.1016/j.taap.2006.12.007
Falzone L, Salomone S, Libra M (2018) Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol 9:1300. https://doi.org/10.3389/fphar.2018.01300
FDA NR (2019) FDA approves new type of therapy to treat advanced urothelial cancer. Mol Cell Pharmacol 11:10–11
Roy A, Wang QJ (2017) Protein kinase D: a potential therapeutic target in prostate cancer. Mol Cell Pharmacol 9:1–4
Jemal A, Torre L, Soerjomataram I, Bray F (2019) The cancer atlas, 3rd edn. American Cancer Society, Atlanta, GA, USA
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
FDA NR (2018) FDA expands approval of imfinzi to reduce the risk of non-small cell lung cancer progressing. Mol Cell Pharmacol 10:1–2
Lilenbaum RC (2019) Overview of the initial treatment of advanced non-small cell lung cancer. In: West H, Vora SR (eds) UpToDate. Wolters Kluwer, Waltham, MA, USA. https://www.uptodate.com. Accessed 23 Oct 2019
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, Patnaik A, Gubens M, Ramalingam SS, Felip E, Goldman JW, Scalzo C, Jensen E, Kush DA, Hui R (2019) Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 37:2518–2527. https://doi.org/10.1200/JCO.19.00934
ClinicalTrial.gov (2020) https://clinicaltrials.gov/. Accessed 25 Jan 2020
Ouyang X, Shi M, Jie F, Bai Y, Shen P, Yu Z, Wang X, Huang C, Tao M, Wang Z, Xie C, Wu Q, Shu Y, Han B, Zhang F, Zhang Y, Hu C, Ma X, Liang Y, Wang A, Lu B, Shi Y, Chen J, Zhuang Z, Wang J, Huang J, Wang C, Bai C, Zhou X, Li Q, Chen F, Yu H, Feng J (2018) Phase III study of dulanermin (recombinant human tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand) combined with vinorelbine and cisplatin in patients with advanced non-small-cell lung cancer. Investig New Drugs 36:315–322. https://doi.org/10.1007/s10637-017-0536-y
Acknowledgements
We thank Dr. Wafik el-Deiry (Brown University) for providing DR5 promoter-luciferase construct. Asma Umer Khayam was supported by a fellowship from Higher Education Commission, Islamabad, Pakistan. This work was supported, in part, by the Carol M. Baldwin Breast Cancer Research Fund and Michael E. Connolly Endowment for Lung Cancer Research, Upstate Cancer Center grant to Dr. M. Saeed Sheikh.
Author information
Authors and Affiliations
Contributions
AUK: Conceptualization; Investigation; Methodology; Validation; Writing—original draft; Writing—review & editing. HP: Investigation; Methodology; Validation; Writing—review & editing. NAF: Methodology; Validation; Writing—review & editing. AEFM: Methodology; Validation; Writing—review & editing. ED: Methodology; Validation; Writing—review & editing. BM: Resources; Supervision; Writing—review & editing. YH: Resources; Supervision; Writing—review & editing. MSS: Conceptualization; Investigation; Funding acquisition; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to declare in relation to the work described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2020_3841_MOESM1_ESM.tif
Supplementary file1 Supplementary Figure 1 QA does not regulate DR5 gene promoter. (A) Schematic of DR5 gene promoter luciferase construct used in this study. (B) QA effect on DR5 promoter activity in A549 lung cancer cells. QA does not upregulate DR5 promoter. In the same experiments, cells were separately treated with thapsigargin (TG), used as a positive control that is known to upregulate DR5 promoter activity. As expected, TG upregulates DR5 promoter activity. QA or TG treatment was for 24 hours. (C) QA does not upregulate DR5 promoter activity in MCF-7 human breast cancer cells. Cells were transiently transfected with DR5 promoter luciferase construct. After ~24 hours, cells were treated with QA or vehicle control for the indicated periods of time. Relative luciferase activity was calculated and plotted as fold induction. The values are presented as mean ± SEM of three independent measurements. (TIF 69 kb)
Rights and permissions
About this article
Cite this article
Khayam, A.U., Patel, H., Faiola, N.A. et al. Quinovic acid purified from medicinal plant Fagonia indica mediates anticancer effects via death receptor 5. Mol Cell Biochem 474, 159–169 (2020). https://doi.org/10.1007/s11010-020-03841-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03841-4