Abstract
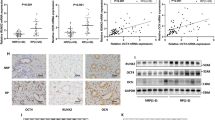
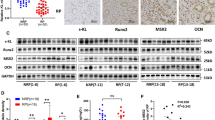
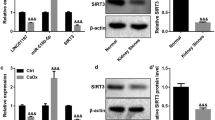
Randall’s plaque (RP) serves as a nidus on which idiopathic calcium oxalate stones form. Renal interstitial mineralization may be the cause underlying RP, and recent studies demonstrated the similarities between the interstitial mineralization and ectopic calcification. The present study aimed to investigate whether human renal interstitial fibroblasts (hRIFs) could form calcification under osteogenic conditions, and whether long non-coding RNA H19 participated in regulating osteogenic differentiation of hRIFs through Wnt-β-catenin pathway. HRIFs were isolated and induced for osteogenic differentiation under osteogenic conditions. Runx2, OCN, alkaline phosphatase (ALP) activity, and the mineralized nodule formation were used to assess the osteogenic phenotype. Molecule expressions were determined by qRT-PCR, immunofluorescence staining, and western blot. The mineralized nodules were assessed by Alizarin red staining. Compared to the normal renal papillary tissue, Runx2, OCN, and H19 were significantly upregulated in RP. After hRIFs were induced with osteogenic medium, osteogenic markers (Runx2, OCN and ALP), β‐catenin and H19 were significantly upregulated, and the mineralized nodules are formed. Additionally, overexpression of H19 promoted the osteogenic phenotype of hRIFs and increased the expression of β‐catenin, whereas knock-down of H19 or XAV939 (inhibitor of Wnt-β‐catenin signaling pathway) significantly repressed the osteogenic phenotype of hRIFs and decreased the β‐catenin. Moreover, XAV939 was shown to abolish the osteogenic differentiation of hRIFs promoted by H19. The study demonstrated that ectopic calcification partly participated in the formation of RP, and H19 promoted osteogenic differentiation of hRIFs by activating Wnt-β-catenin pathway, which shed new light on the molecular mechanism of the RP formation.




Similar content being viewed by others
Data availability
The data will not be deposited. Please contact the corresponding author if required.
Change history
07 July 2020
In the original article, Fig.��2c was published incorrectly. The correct version of Fig.��2c is provided in this correction.
References
Turney BW, Reynard JM, Noble JG, Keoghane SR (2012) Trends in urological stone disease. BJU Int 109(7):1082. https://doi.org/10.1111/j.1464-410X.2011.10495.x
Neisius A, Preminger GM (2013) Epidemiology, prevention and redefining therapeutic standards. Nat Rev Urol 10(2):75–77. https://doi.org/10.1038/nrurol.2012.253
Heers H, Turney BW (2016) Trends in urological stone disease: a 5-year update of hospital episode statistics. BJU Int 118(5):785–789. https://doi.org/10.1111/bju.13520
Uribarri J (2020) Chronic kidney disease and kidney stones. Curr Opin Nephrol Hypertens 29(2):237–242. https://doi.org/10.1097/mnh.0000000000000582
Randall A (1937) The origin and growth of renal calculi. Ann Surg 105(6):1009–1027. https://doi.org/10.1097/00000658-193706000-00014
Hsi RS, Ramaswamy K, Ho SP, Stoller ML (2017) The origins of urinary stone disease: upstream mineral formations initiate downstream Randall's plaque. BJU Int 119(1):177–184. https://doi.org/10.1111/bju.13555
Evan AP, Coe FL, Lingeman JE et al (2007) Mechanism of formation of human calcium oxalate renal stones on Randall's plaque. Anat Record Adv Integr Anat Evolut Biol 290(10):1315–1323. https://doi.org/10.1002/ar.20580
Mezzabotta F, Cristofaro R, Ceol M et al (2015) Spontaneous calcification process in primary renal cells from a medullary sponge kidney patient harbouring a GDNF mutation. J Cell Mol Med 19(4):889–902. https://doi.org/10.1111/jcmm.12514
Sharpe CC, Dockrell ME (2012) Primary culture of human renal proximal tubule epithelial cells and interstitial fibroblasts. Methods Mole Biol (Clifton, NJ) 806:175–185. https://doi.org/10.1007/978-1-61779-367-7_12
de Vries TJ, Schoenmaker T, Micha D et al (2018) Periodontal ligament fibroblasts as a cell model to study osteogenesis and osteoclastogenesis in fibrodysplasia ossificans progressiva. Bone 109:168–177. https://doi.org/10.1016/j.bone.2017.07.007
Dunham I, Kundaje A, Aldred SF et al (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489(7414):57–74. https://doi.org/10.1038/nature11247
Brosnan CA, Voinnet O (2009) The long and the short of noncoding RNAs. Curr Opin Cell Biol 21(3):416–425. https://doi.org/10.1016/j.ceb.2009.04.001
Iyer MK, Niknafs YS, Malik R et al (2015) The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 47(3):199–208. https://doi.org/10.1038/ng.3192
Kapranov P, Cheng J, Dike S et al (2007) RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science (New York, NY) 316(5830):1484–1488. https://doi.org/10.1126/science.1138341
Ju C, Liu R, Zhang Y-W et al (2019) Mesenchymal stem cell-associated lncRNA in osteogenic differentiation. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2019.108912
Boudin E, Fijalkowski I, Piters E, Van Hul W (2013) The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum 43(2):220–240. https://doi.org/10.1016/j.semarthrit.2013.01.004
Jin H, Wang B, Li J et al (2015) Anti-DKK1 antibody promotes bone fracture healing through activation of beta-catenin signaling. Bone 71:63–75. https://doi.org/10.1016/j.bone.2014.07.039
Gong YY, Peng MY, Yin DQ, Yang YF (2018) Long non-coding RNA H19 promotes the osteogenic differentiation of rat ectomesenchymal stem cells via Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci 22(24):8805–8813. https://doi.org/10.26355/eurrev_201812_16648
Zhou P, Li Y, Di R, Yang Y, Meng S, Song F, Ma L (2019) H19 and Foxc2 synergistically promotes osteogenic differentiation of BMSCs via Wnt-beta-catenin pathway. J Cell Physiol 234(8):13799–13806. https://doi.org/10.1002/jcp.28060
Rodemann HP, Muller GA, Knecht A, Norman JT, Fine LG (1991) Fibroblasts of rabbit kidney in culture. I. Characterization and identification of cell-specific markers. Am J Physiol 261(2 Pt 2):F283–F291. https://doi.org/10.1152/ajprenal.1991.261.2.F283
Evan AP (2010) Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol (Berlin, Germany) 25(5):831–841. https://doi.org/10.1007/s00467-009-1116-y
Evan AP, Coe FL, Lingeman JE, Worcester E (2005) Insights on the pathology of kidney stone formation. Urol Res 33(5):383–389. https://doi.org/10.1007/s00240-005-0488-0
Joshi S, Clapp WL, Wang W (1852) Khan SR (2015) Osteogenic changes in kidneys of hyperoxaluric rats. Biochim et Biophys Acta Mol Basis Dis 9:2000–2012. https://doi.org/10.1016/j.bbadis.2015.06.020
Khan SR, Rodriguez DE, Gower LB, Monga M (2012) Association of Randall plaque with collagen fibers and membrane vesicles. J Urol 187(3):1094–1100. https://doi.org/10.1016/j.juro.2011.10.125
Miyazawa K, Aihara K, Ikeda R, Moriyama MT, Suzuki K (2009) cDNA macroarray analysis of genes in renal epithelial cells exposed to calcium oxalate crystals. Urol Res 37(1):27–33. https://doi.org/10.1007/s00240-008-0164-2
Peerapen P, Thongboonkerd V (2011) Effects of calcium oxalate monohydrate crystals on expression and function of tight junction of renal tubular epithelial cells. Lab Investig 91(1):97–105. https://doi.org/10.1038/labinvest.2010.167
Kumar V, Farell G, Yu S et al (2006) Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med 54(7):412–424. https://doi.org/10.2310/6650.2006.06021
Priante G, Ceol M, Gianesello L, Furlan C, Del Prete D, Anglani F (2019) Human proximal tubular cells can form calcium phosphate deposits in osteogenic culture: role of cell death and osteoblast-like transdifferentiation. Cell Death Discov 5:57. https://doi.org/10.1038/s41420-019-0138-x
Priante G, Quaggio F, Gianesello L et al (2018) Caspase-independent programmed cell death triggers Ca2PO4 deposition in an in vitro model of nephrocalcinosis. Biosci Rep. https://doi.org/10.1042/bsr20171228
Priante G, Mezzabotta F, Cristofaro R et al (2019) Cell death in ectopic calcification of the kidney. Cell Death Dis 10(6):466. https://doi.org/10.1038/s41419-019-1697-8
Gambaro G, D'Angelo A, Fabris A, Tosetto E, Anglani F, Lupo A (2004) Crystals, Randall's plaques and renal stones: do bone and atherosclerosis teach us something? J Nephrol 17(6):774–777. https://doi.org/10.5772/intechopen.69895
Priante G, Ceol M, Terrin L, Gianesello L, Quaggio F, Del Prete D, Anglani F (2017) Understanding the pathophysiology of nephrocalcinosis. Intechopen, London
Yoshimura H, Matsuda Y, Yamamoto M, Kamiya S, Ishiwata T (2018) Expression and role of long non-coding RNA H19 in carcinogenesis. Front Biosci Landmark 23:614–625. https://doi.org/10.2741/4608
Wang Y, Liu W, Liu Y et al (2018) Long noncoding RNA H19 mediates LCoR to impact the osteogenic and adipogenic differentiation of mBMSCs in mice through sponging miR-188. J Cell Physiol 233(9):7435–7446. https://doi.org/10.1002/jcp.26589
Wu J, Zhao J, Sun L, Pan Y, Wang H, Zhang WB (2018) Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone 108:62–70. https://doi.org/10.1016/j.bone.2017.12.013
Gao X, Ge J, Li W, Zhou W, Xu L (2018) LncRNA KCNQ1OT1 promotes osteogenic differentiation to relieve osteolysis via Wnt/beta-catenin activation. Cell Biosci 8:19. https://doi.org/10.1186/s13578-018-0216-4
Hu K, Jiang W, Sun H, Li Z, Rong G, Yin Z (2019) Long noncoding RNA ZBED3-AS1 induces the differentiation of mesenchymal stem cells and enhances bone regeneration by repressing IL-1beta via Wnt/beta-catenin signaling pathway. J Cell Physiol 234(10):17863–17875. https://doi.org/10.1002/jcp.28416
Deng L, Hong H, Zhang X, Chen D, Chen Z, Ling J, Wu L (2018) Down-regulated lncRNA MEG3 promotes osteogenic differentiation of human dental follicle stem cells by epigenetically regulating Wnt pathway. Biochem Biophys Res Commun 503(3):2061–2067. https://doi.org/10.1016/j.bbrc.2018.07.160
Taguchi K, Hamamoto S, Okada A et al (2017) Genome-wide gene expression profiling of randall's plaques in calcium oxalate stone formers. J Am Soc Nephrol JASN 28(1):333–347. https://doi.org/10.1681/asn.2015111271
Zhang C, Yuan J, Hu H et al (2017) Long non-coding RNA CHCHD4P4 promotes epithelial-mesenchymal transition and inhibits cell proliferation in calcium oxalate-induced kidney damage. Braz J Med Biol Res 51(1):e6536. https://doi.org/10.1590/1414-431X20176536
Chen J, Zhang D, Ji M-F, Liu T, Mei C-l, Tang X-J (2019) Activation of liver X receptor suppresses osteopontin expression and ameliorates nephrolithiasis. J Cell Physiol 234(8):14109–14122. https://doi.org/10.1002/jcp.28101
Acknowledgements
We are grateful for the funding provided by the Nature Science Foundation of Hunan province, China 2017JJ2395 (to Yang Li).
Funding
This study was funded by the Nature Science Foundation of Hunan province, China (2017JJ2395).
Author information
Authors and Affiliations
Contributions
Conceptualization: ZZ, YC; Methodology: FH, YC, FZ; Formal analysis and investigation: ZZ, HZ, WX; Writing-original draft preparation: ZZ; Writing-review and editing: YC, JC; Funding acquisition: YL; Resources: ZC, CH; Supervision: HC, YL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Xiangya Hospital of Central South University (Date: July 23, 2017 /No.201705768).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, Z., Cui, Y., Huang, F. et al. Long non-coding RNA H19 promotes osteogenic differentiation of renal interstitial fibroblasts through Wnt-β-catenin pathway. Mol Cell Biochem 470, 145–155 (2020). https://doi.org/10.1007/s11010-020-03753-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03753-3