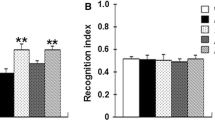
Abstract
Alzheimer's disease (AD) is one of the most serious neurodegenerative diseases and is characterized by progressive cognitive impairment and multiple neurological changes. To date, there are no effective drugs to delay or cure AD. Breviscapine (Bre) is an active ingredient of flavonoids extracted from breviscapus. Previous research suggests that Bre is an effective medicine for the prevention and treatment of AD. In the present study, we sought to explore the molecular mechanisms responsible for short-term beneficial effects of Breviscapine on Aβ burden, neuronal and synaptic, cognitive function in APP/PS1 transgenic mice at 6 months age. Our results showed that 3 months of intraperitoneal treatment with Bre rescued learning deficits, relieved memory retention, improved the ability to explore the outside world, markedly decreased Aβ burden, attenuated function of neocortical and hippocampal neuron, and increased the synaptic proteins levels in the brain of APP/PS1 mice by decreasing BACE1, promoting Aβ-degrading enzyme IDE expression, suppressing RAGE expression, and regulating p38/p53/NT4 pathway. This finding provides more evidence of neuroprotective effects and action mechanisms of Bre antagonist AD, suggesting that Bre may have potential as anti-AD agent.






Similar content being viewed by others
References
The L (2016) Alzheimer's disease: expedition into the unknown. Lancet 4:388–2713. https://doi.org/10.1016/S0140-6736(16)32457-6
Jiang T, Chang RC, Rosenmann H, Yu JT (2015) Advances in alzheimer's disease: from bench to bedside. Biomed Res Int 11:202–676. https://doi.org/10.1155/2015/202676
Uddin MS, Kabir MT, Al Mamun A, Abdel-Daim MM, Barreto GE, Ashraf GM (2019) Apoe and alzheimer's disease: evidence mounts that targeting apoe4 may combat alzheimer's pathogenesis. Mol Neurobiol 56:2450–2465. https://doi.org/10.1007/s12035-018-1237-z
Gustavsson A, Green C, Jones RW, Förstl H, Simsek D, de Reydet de Vulpillieres F, Luthman S, Adlard N, Bhattacharyya S, Wimo A (2017) Current issues and future research priorities for health economic modelling across the full continuum of alzheimer's disease. Alzheimers Dement 13:312–321. https://doi.org/10.1016/j.jalz.2016.12.005
Chinese Pharmacopoeia Commission (2010) Pharmacopoeia of the People's Republic of China. China Medical Science and Technology Press part 1:138
Zhang WD, Chen WS, Kong DY (2000) Study on chemical composition of herba Erigeron breviscapus (I). J Second Mil Med Univ 2:143–145. https://doi.org/10.16781/j.0258-879x.2000.02.022
Yan YZ (2008) Research progress on the protective mechanism of breviscapine on cerebral ischemia reperfusion injury. Sichuan J Physiol Sci 30:129–130.
Shi YH, Miao H, Su PF (2008) Effect of breviscapine on memory impairment induced by d-galactose in mice. Lishizhen Med Mater Med Res 19:1445–1446. https://doi.org/10.3969/j.issn.1008-0805.2008.06.077
Mao JQ, Qiu Y, Li TJ (2017) Effects of breviscapine on learning and memory function in rats with traumatic brain injury. Chin J Pharm 23(167–169):240. https://doi.org/10.3969/j.issn.1008-9926.2007.03.003
Zeng YQ, Cui YB, Gu JH, Liang C, Zhou XF (2018) Scutellarin mitigates abeta-induced neurotoxicity and improves behavior impairments in ad mice. Molecules 9:23. https://doi.org/10.3390/molecules23040869
Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR (2001) Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng 17:157–165. https://doi.org/10.1016/s1389-0344(01)00067-3
Malm T, Koistinaho J, Kanninen K (2011) Utilization of APPswe/PS1dE9 transgenic mice in research of alzheimer's disease: focus on gene therapy and cell-based therapy applications. Int J Alzheimers Dis 2011:517160. https://doi.org/10.4061/2011/517160
Zeng YQ, Wang YJ, Zhou XF (2014) Effects of (-) epicatechin on the pathology of app/ps1 transgenic mice. Front Neurol 5:69. https://doi.org/10.3389/fneur.2014.00069
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M (2010) Alzheimer's disease: clinical trials and drug development. Lancet Neurol 9:702–716. https://doi.org/10.1016/S1474-4422(10)70119-8
Schonrock N, Matamales M, Ittner LM, Götz J (2012) Microrna networks surrounding app and amyloid-beta metabolism–implications for alzheimer's disease. Exp Neurol 235:447–454. https://doi.org/10.1016/j.expneurol.2011.11.013
Haass C, Selkoe DJ (2017) Soluble protein oligomers in neurodegeneration: lessons from the alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–112. https://doi.org/10.1038/nrm2101
Kobayashi D, Zeller M, Cole T, Buttini M, McConlogue L, Sinha S, Freedman S, Morris RG, Chen KS (2008) Bace1 gene deletion: impact on behavioral function in a model of alzheimer's disease. Neurobiol Aging 29:861–873. https://doi.org/10.1016/j.neurobiolaging.2007.01.002
Probst G, Xu YZ (2012) Small-molecule bace1 inhibitors: a patent literature review (2006–2011). Expert Opin Ther Pat 22:511–540. https://doi.org/10.1517/13543776.2012.681302
Yu SL, Wong CK, Szeto CC, Li EK, Cai Z, Tam LS (2015) Members of the receptor for advanced glycation end products axis as potential therapeutic targets in patients with lupus nephritis. Lupus 24:675–686. https://doi.org/10.1177/0961203314559631
Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP (2005) Understanding rage, the receptor for advanced glycation end products. J Mol Med (Berl) 83:876–886. https://doi.org/10.1007/s00109-005-0688-7
Wan W, Cao L, Liu L, Zhang C, Kalionis B, Tai X, Li Y, Xia S (2015) Abeta(1–42) oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of rage and metalloproteinases, and down-regulation of tight junction scaffold proteins. J Neurochem 134:382–393. https://doi.org/10.1111/jnc.13122
Deane R, Du YS, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B (2003) Rage mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9:907–913. https://doi.org/10.1038/nm890
Gilbert BJ (2013) The role of amyloid beta in the pathogenesis of alzheimer's disease. J Clin Pathol 66:362–366. https://doi.org/10.1136/jclinpath-2013-201515
Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ (2003) Enhanced proteolysis of beta-amyloid in app transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40:1087–1093. https://doi.org/10.1016/s0896-6273(03)00787-6
Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA 100:4162–4167. https://doi.org/10.1073/pnas.0230450100
Carew TJ (1996) Molecular enhancement of memory formation. Neuron 16:5–8. https://doi.org/10.1016/s0896-6273(00)80016-1
Reustle A, Torzewski M (2018) Role of p38 mapk in atherosclerosis and aortic valve sclerosis. Int J Mol Sci. https://doi.org/10.3390/ijms19123761
Roy S, Rana A, Akhter Y, Hande MP, Banerjee B (2018) The role of p38 mapk pathway in p53 compromised state and telomere mediated DNA damage response. Mutat Res Genet Toxicol Environ Mutagen 836:89–97. https://doi.org/10.1016/j.mrgentox.2018.05.018
Mattson MP (2004) Pathways towards and away from alzheimer's disease. Nature 430:631–639. https://doi.org/10.1038/nature02621
LaFerla FM, Hall CK, Ngo L, Jay G (1996) Extracellular deposition of beta-amyloid upon p53-dependent neuronal cell death in transgenic mice. J Clin Invest 98:1626–1632. https://doi.org/10.1172/JCI118957
Lapresa R, Agulla J, Sanchez-Moran I, Zamarreño R, Prieto E, Bolaños JP, Almeida A (2019) Amyloid-ss promotes neurotoxicity by cdk5-induced p53 stabilization. Neuropharmacology 146:19–27. https://doi.org/10.1016/j.neuropharm.2018.11.019
Hou Y, Aboukhatwa MA, Lei DL, Manaye K, Khan I, Luo Y (2010) Anti-depressant natural flavonols modulate bdnf and beta amyloid in neurons and hippocampus of double tgad mice. Neuropharmacology 58:911–920. https://doi.org/10.1016/j.neuropharm.2009.11.002
Cotman CW (2005) The role of neurotrophins in brain aging: a perspective in honor of regino perez-polo. Neurochem Res 30:877–881. https://doi.org/10.1007/s11064-005-6960-y
Acknowledgements
This work was supported by Yunnan Biopharmaceutical Major Project (2018ZF002), Union Foundation of Yunnan Applied Research Project 2017FE468(-183), and Innovation Team Project of Yunnan Education Department.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicst of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, XB., Gu, JH. et al. Breviscapine exerts neuroprotective effects through multiple mechanisms in APP/PS1 transgenic mice. Mol Cell Biochem 468, 1–11 (2020). https://doi.org/10.1007/s11010-020-03698-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03698-7