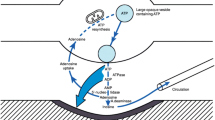
Abstract
We reported previously that the rat submandibular gland is able to release nanovesicles capable to hydrolyse millimolar concentrations of ATP, ADP and AMP in vitro. Here, we show that rat saliva also contains nanovesicles with the ability to hydrolyse ATP. Our aim was to identify and characterize vesicular nucleotidases by using kinetic, immunological and in silico approaches. Nucleotidase activity in the absence or presence of specific inhibitors allowed us to assume the participation of NTPDase1, -2 and -3, together with ecto-5′-nucleotidase, confirmed using specific antibodies. At neutral pH, initial ATPase activity would be mostly due to NTPDase2, which was thereafter inactivated, leaving NTPDase1 and NTPDase3 to hydrolyse ATP and ADP with an efficacy ATPase/ADPase around 2. Ecto-5′nucleotidase would be mainly responsible for AMP hydrolysis and adenosine accumulation. We proposed a kinetic model for NTPDase2 as a tool to isolate and analyse the turnover of this enzyme in the presence of different ATP concentrations, including those expected in extracellular media. Our study characterizes the ectonucleotidases carried by extracellular vesicles which contribute to modulate ATP and adenosine concentrations in the oral cavity, essential players in purinergic signalling.








Similar content being viewed by others
References
Han Y, Jia L, Zheng Y, Li W (2018) Salivary exosomes: emerging roles in systemic disease. Int J Biol Sci 14(6):633–643
González D, Egido P, Balcarcel N, Hattab C, Barbieri van Haaster M, Pelletier J, Sévigny J, Ostuni M (2015) Rat submandibular glands secrete nanovesicles with NTPDase and 5′-nucleotidase activities. Purinergic Signal 11(1):107–116
González D, Barbieri van Haaster M, Quinteros Villarruel E, Brandt M, Benítez M, Stranieri G, Orman B (2018) Histamine stimulates secretion of extracellular vesicles with nucleotidase activity in rat submandibular gland. Arch Oral Biol 85:201–206
Burnstock G, Knight G (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304
Lee M, Zeng W, Muallem S (1997) Characterization and localization of P2 receptors in rat submandibular gland acinar and duct cells. J Biol Chem 272(52):32951–32955
Turner J, Landon L, Gibbons S, Talamo B (1999) Salivary gland P2 nucleotide receptors. Crit Rev Oral Biol Med 10(2):210–224
Fontanils U et al (1800) Stimulation by P2X(7) receptors of calcium-dependent production of reactive oxygen species (ROS) in rat submandibular glands. Biochim Biophys Acta 11:1183–1191
Nakamoto T, Brown D, Catalan M, Gonzalez-Begne M, Romanenko V, Melvin J (2009) Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J Biol Chem 284:4815–4822
Woods L, Camden J, Batek J, Petris M, Erb L, Weisman G (2012) P2X7 receptor activation induces inflammatory responses in salivary gland epithelium. Am J Physiol Cell Physiol 303:790–801
Gallez F, Fadel M, Scruel O, Cantraine F, Courtois P (2000) Salivary biomass assessed by bioluminescence ATP assay related to (bacterial and somatic) cell counts. Cell Biochem Funct 18(2):103–108
Lim JC, Mitchell CH (2012) Inflammation, pain, and pressure–purinergic signaling in oral tissues. J Dent Res 91(12):1103–1109
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16(3):177–192
Kukulski F et al (2005) Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal 1(2):193–204
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8(3):437–502
Yegutkin G (2014) Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol 49(6):473–497
Knowles A (2011) The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal 7(1):21–45
Baginski E, Foà P, Zak B (1967) Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem 13(4):326–332
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3(3):22
Hecht JP, Nikonov JM, Alonso GL (1990) A BASIC program for the numerical solution of the transient kinetics of complex biochemical models. Comput Methods Programs Biomed 33(1):13–20
Kozlenkov A, Le Du MH, Cuniasse P, Ny T, Hoylaerts MF, Millán JL (2004) Residues determining the binding specificity of uncompetitive inhibitors to tissue-nonspecific alkaline phosphatase. J Bone Miner Res 19(11):1862–1872
Iqbal J, Vollmayer P, Braun N, Zimmermann H, Müller C (2005) A capillary electrophoresis method for the characterization of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and the analysis of inhibitors by in-capillary enzymatic microreaction. Purinergic Signal 1(4):349–358
Iqbal J, Lévesque S, Sévigny J, Müller C (2008) A highly sensitive CE-UV method with dynamic coating of silica-fused capillaries for monitoring of nucleotide pyrophosphatase/phosphodiesterase reactions. Electrophoresis 29(17):3685–3693
Joseph S, Pifer M, Przybylski R, Dubyak G (2004) Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol 142(6):1002–1014
Araujo C, Quintero I, Kipar A, Herrala A, Pulkka A, Saarinen L, Sampsa H, Vihko P (2014) Prostatic acid phosphatase is the main acid phosphatase with 5′-ectonucleotidase activity in the male mouse saliva and regulates salivation. Am J Physiol Cell Physiol 306(11):1017–1027
Panula P, Chazot P, Cowart M, Gutzmer R, Leurs R, Liu W, Haas H (2015) International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67(3):601–655
Kittel A et al (2004) Localization of nucleoside triphosphate diphosphohydrolase-1 (NTPDase1) and NTPDase2 in pancreas and salivary gland. J Histochem Cytochem 52(7):861–871
Lavoie E, Gulbransen B, Martín-Satué M, Aliagas E, Sharkey K, Sévigny J (2011) Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am J Physiol Gastrointest Liver Physiol 300(4):608–620
Ishibashi K, Kamura K, Yamazaki J (2007) Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl(−) reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol 294(5):1729–1736
Henz S, Ribeiro C, Rosa A, Chiarelli R, Casali E, Sarkis J (2006) Kinetic characterization of ATP diphosphohydrolase and 5′-nucleotidase activities in cells cultured from submandibular salivary glands of rats. Cell Biol Int 30(3):214–220
Henz S, Fürstenau C, Chiarelli R, Sarkis J (2007) Kinetic and biochemical characterization of an ecto-nucleotide pyrophosphatase/phosphodiesterase (EC 3.1.4.1) in cells cultured from submandibular salivary glands of rats. Arch Oral Biol 52(10):916–923
Beeler T, Wang T, Gable K, Lee S (1985) Comparison of the rat microsomal Mg-ATPase of various tissues. Arch Biochem Biophys 243(2):644–654
Knowles A, Chiang W (2003) Enzymatic and transcriptional regulation of human ecto-ATPase/E-NTPDase 2. Arch Biochem Biophys 418(2):217–227
Martín-Romero F, García-Martín E, Gutiérrez-Merino C (1996) Inactivation of ecto-ATPase activity of rat brain synaptosomes. Biochim Biophys Acta 1283(1):51–59
Vlajkovi S, Wang C, Soeller C, Zimmermann H, Thorne P, Housley G (2007) Activation-dependent trafficking of NTPDase2 in Chinese hamster ovary cells. Int J Biochem Cell Biol 39(4):810–817
Jiang Z, Wu Y, Csizmadia E, Feldbrügge L, Enjyoji K, Tigges J, Robson S (2014) Characterization of circulating microparticle-associated CD39 family ecto-nucleotidases in human plasma. Purinergic Signal 10(4):611–618
Clayton A, Al-Taei S, Webber J, Mason M, Tabi Z (2011) Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 187(2):676–683
Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, Tabi Z (2017) Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles 6(1):1368823
Fan W, Wang W, Wu J, Ma L, Guo J (2017) Identification of CD4+ T-cell-derived CD161+ CD39+ and CD39+ CD73+ microparticles as new biomarkers for rheumatoid arthritis. Biomark Med 11(2):107–116
Ceruti S, Colombo L, Magni G, Viganò F, Boccazzi M, Deli M, Kittel A (2011) Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood–brain barrier. Neurochem Int 59(2):259–271
Binderman I, Gadban N, Yaffe A (2017) Extracellular ATP is a key modulator of alveolar bone loss in periodontitis. Arch Oral Biol 81:131–135
Armstrong FB (1989) Biochemistry, 3rd edn. Oxford University Press, New York
Kukulski F, Komoszyński M (2003) Purification and characterization of NTPDase1 (ecto-apyrase) and NTPDase2 (ecto-ATPase) from porcine brain cortex synaptosomes, (in eng). Eur J Biochem 270(16):3447–3454
Teruel JA, Kurzmack M, Inesi G (1987) Kinetic and thermodynamic control of ATP synthesis by sarcoplasmic reticulum adenosinetriphosphatase. J Biol Chem 262(27):13055–13060
Grubmeyer C, Cross RL, Penefsky HS (1982) Mechanism of ATP hydrolysis by beef heart mitochondrial ATPase. Rate constants for elementary steps in catalysis at a single site. J Biol Chem 257(20):12092–12100
Stout JG, Kirley TL (1994) Purification and characterization of the ecto-Mg-ATPase of chicken gizzard smooth muscle. J Biochem Biophys Methods 29(1):61–75
Treuheit MJ, Vaghy PL, Kirley TL (1992) Mg(2+)-ATPase from rabbit skeletal muscle transverse tubules is 67-kilodalton glycoprotein. J Biol Chem 267(17):11777–11782
Acknowledgements
This work was supported by the Universidad de Buenos Aires (UBACYT Grants 2002015020012BA and 20720150100006BA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González, D.A., Barbieri van Haaster, M.M., Quinteros Villarruel, E. et al. Salivary extracellular vesicles can modulate purinergic signalling in oral tissues by combined ectonucleoside triphosphate diphosphohydrolases and ecto-5′-nucleotidase activities. Mol Cell Biochem 463, 1–11 (2020). https://doi.org/10.1007/s11010-019-03624-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03624-6