Abstract
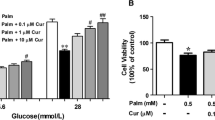
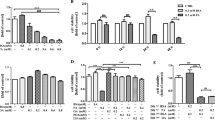
High levels of circulating free fatty acids often trigger pancreatic β cell dysfunction during the development of type 2 diabetes. Silibinin, the main component of Silybum marianum fruit extract (silymarin), is reported to have anti-diabetic effect. This study is designed to determine the protective effect of silibinin on palmitic acid-induced damage in a rat pancreatic β-cell line, INS-1 cells. Our results demonstrate that silibinin improves cell viability, enhances insulin synthesis and secretion, and resumes normal mitochondrial function in palmitic acid-treated INS-1 cells. An accumulating body of evidence has shown that the estrogen receptors are key molecules involved in glucose and lipid metabolism. Our results suggest that silibinin upregulates ERα signaling pathway from the finding that ERα-specific inhibitors abolish the anti-lipotoxic effect of silibinin. In conclusion, these findings suggest that silibinin protects INS-1 cells against apoptosis and mitochondrial damage through upregulation of ERα pathway.





Similar content being viewed by others
References
Zheng Y, Ley SH, Hu FB (2017) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98
Fex M, Nicholas LM, Vishnu N, Medina A, Sharoyko VV, Nicholls DG, Spegel P, Mulder H (2018) The pathogenetic role of beta-cell mitochondria in type 2 diabetes. J Endocrinol 236(3):R145–R159. https://doi.org/10.1530/JOE-17-0367
Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M (2012) National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr 10(1):22
Lee E, Choi J, Lee HS (2017) Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J Cell Physiol 232(12):3209–3217. https://doi.org/10.1002/jcp.25867
Tuo Y, Wang D, Li S, Chen C (2011) Long-term exposure of INS-1 rat insulinoma cells to linoleic acid and glucose in vitro affects cell viability and function through mitochondrial-mediated pathways. Endocrine 39(2):128–138. https://doi.org/10.1007/s12020-010-9432-3
Ciregia F, Bugliani M, Ronci M, Giusti L, Boldrini C, Mazzoni MR, Mossuto S, Grano F, Cnop M, Marselli L, Giannaccini G, Urbani A, Lucacchini A, Marchetti P (2017) Palmitate-induced lipotoxicity alters acetylation of multiple proteins in clonal beta cells and human pancreatic islets. Sci Rep 7(1):13445. https://doi.org/10.1038/s41598-017-13908-w
Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB (2009) The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 304(1–2):63–68. https://doi.org/10.1016/j.mce.2009.02.016
Burns KA, Korach KS (2012) Estrogen receptors and human disease: an update. Arch Toxicol 86(10):1491–1504. https://doi.org/10.1007/s00204-012-0868-5
Jia G, Aroor AR, Sowers JR (2014) Estrogen and mitochondria function in cardiorenal metabolic syndrome. Prog Mol Biol Transl Sci 127:229–249. https://doi.org/10.1016/B978-0-12-394625-6.00009-X
Hamilton DJ, Minze LJ, Kumar T, Cao TN, Lyon CJ, Geiger PC, Hsueh WA, Gupte AA (2016) Estrogen receptor alpha activation enhances mitochondrial function and systemic metabolism in high-fat-fed ovariectomized mice. Physiol Rep 4(17):e12913. https://doi.org/10.14814/phy2.12913
Mauvais-Jarvis F (2011) Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 22(1):24–33. https://doi.org/10.1016/j.tem.2010.10.002
Zhou Z, Ribas V, Rajbhandari P, Drew BG, Moore TM, Fluitt AH, Reddish BR, Whitney KA, Georgia S, Vergnes L, Reue K, Liesa M, Shirihai O, van der Bliek AM, Chi NW, Mahata SK, Tiano JP, Hewitt SC, Tontonoz P, Korach KS, Mauvais-Jarvis F, Hevener AL (2018) Estrogen receptor alpha protects pancreatic beta-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J Biol Chem 293(13):4735–4751. https://doi.org/10.1074/jbc.M117.805069
Geisler JG, Walter Z, Kathleen Z, Lakey JRT, Hans S, Milici AJ, Soeller WC (2002) Estrogen can prevent or reverse obesity and diabetes in mice expressing human islet amyloid polypeptide. Diabetes 51(7):2158
Pierre G, Bourgeois EA, Anais L, Linh P, Elodie R, Marie-Louise A, Diane D, Jean-Marc G, Francis B, Claes O (2016) Estrogen therapy delays autoimmune diabetes and promotes the protective efficiency of natural killer T-cell activation in female nonobese diabetic mice. Endocrinology 157(1):258–267
Horn PA, Mohlig M, Osterhoff M, Wolter S, Hofmann J, Stocking C, Ostertag W, Wahl M, Schatz H, Pfeiffer A (2000) Effect of estradiol on insulin secreting INS-1 cells overexpressing estrogen receptors. Eur J Endocrinol 142(1):84
Chen JQ, Yager JD, Russo J (2005) Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta 1746(1):1–17. https://doi.org/10.1016/j.bbamcr.2005.08.001
Wiederkehr A, Wollheim CB (2012) Mitochondrial signals drive insulin secretion in the pancreatic beta-cell. Mol Cell Endocrinol 353(1–2):128–137. https://doi.org/10.1016/j.mce.2011.07.016
Cho YM, Park KS, Lee HK (2007) Genetic factors related to mitochondrial function and risk of diabetes mellitus. Diabetes Res Clin Pract 77(Suppl 1):S172–177. https://doi.org/10.1016/j.diabres.2007.01.052
Belitz AR, Sams CE (2007) Effect of population density on growth, yield, and flavonolignan content in milk thistle (Silybum marianum). Acta Hortic 756(756):251–257
Nejati-Koshki K, Zarghami N, Pourhassan-Moghaddam M, Rahmati-Yamchi M, Mollazade M, Nasiri M, Esfahlan RJ, Barkhordari A, Tayefi-Nasrabadi H (2012) Inhibition of leptin gene expression and secretion by silibinin: possible role of estrogen receptors. Cytotechnology 64(6):719–726. https://doi.org/10.1007/s10616-012-9452-3
Pliskova M, Vondracek J, Kren V, Gazak R, Sedmera P, Walterova D, Psotova J, Simanek V, Machala M (2005) Effects of silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology 215(1–2):80–89. https://doi.org/10.1016/j.tox.2005.06.020
Jahanafrooz Z, Motamed N, Rinner B, Mokhtarzadeh A, Baradaran B (2018) Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci 213:236–247. https://doi.org/10.1016/j.lfs.2018.10.009
Bijak M (2017) Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—chemistry, bioavailability, and metabolism. Molecules 22(11):1942. https://doi.org/10.3390/molecules22111942
Chen K, Zhao L, He H, Wan X, Wang F, Mo Z (2014) Silibinin protects beta cells from glucotoxicity through regulation of the Insig-1/SREBP-1c pathway. Int J Mol Med 34(4):1073–1080. https://doi.org/10.3892/ijmm.2014.1883
Yang J, Sun Y, Xu F, Liu W, Hayashi T, Onodera S, Tashiro SI, Ikejima T (2018) Involvement of estrogen receptors in silibinin protection of pancreatic beta-cells from TNFalpha- or IL-1beta-induced cytotoxicity. Biomed Pharmacother 102:344–353. https://doi.org/10.1016/j.biopha.2018.01.128
Li F, Munsey TS, Sivaprasadarao A (2017) TRPM2-mediated rise in mitochondrial Zn(2 +) promotes palmitate-induced mitochondrial fission and pancreatic beta-cell death in rodents. Cell Death Differ 24(12):1999–2012. https://doi.org/10.1038/cdd.2017.118
Oh YS, Bae GD, Baek DJ (2018) Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front Endocrinol 9:384
Ma ZJ, Lu L, Yang JJ, Wang XX, Su G, Wang ZL, Chen GH, Sun HM, Wang MY, Yang Y (2018) Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur J Pharmacol 821:1–10. https://doi.org/10.1016/j.ejphar.2017.12.027
Jiang L, Liu Y, Ma MM, Tang YB, Zhou JG, Guan YY (2013) Mitochondria dependent pathway is involved in the protective effect of bestrophin-3 on hydrogen peroxide-induced apoptosis in basilar artery smooth muscle cells. Apoptosis 18(5):556–565. https://doi.org/10.1007/s10495-013-0828-4
Tao S, Ren Y, Zheng H, Zhao M, Zhang X, Zhu Y, Yang J, Zheng S (2017) Salvianolic acid B inhibits intermittent high glucose-induced INS-1 cell apoptosis through regulation of Bcl-2 proteins and mitochondrial membrane potential. Eur J Pharmacol 814:56–62. https://doi.org/10.1016/j.ejphar.2017.08.007
Adzic M, Mitic M, Radojcic M (2017) Mitochondrial estrogen receptors as a vulnerability factor of chronic stress and mediator of fluoxetine treatment in female and male rat hippocampus. Brain Res 1671:77–84. https://doi.org/10.1016/j.brainres.2017.07.007
Liao TL, Tzeng CR, Yu CL, Wang YP, Kao SH (2015) Estrogen receptor-beta in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis. Ann N Y Acad Sci 1350:52–60. https://doi.org/10.1111/nyas.12872
Huang CN, Wang CJ, Lee YJ, Peng CH (2017) Active subfractions of Abelmoschus esculentus substantially prevent free fatty acid-induced beta cell apoptosis via inhibiting dipeptidyl peptidase-4. PLoS ONE 12(7):e0180285. https://doi.org/10.1371/journal.pone.0180285
Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS (2017) Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med 49(2):e291. https://doi.org/10.1038/emm.2016.157
Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, Ye T, Kang M, Shen H, Dong S (2013) Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis 4:e460. https://doi.org/10.1038/cddis.2012.206
Xu F, Yang J, Negishi H, Sun Y, Li D, Zhang X, Hayashi T, Gao M, Ikeda K, Ikejima T (2018) Silibinin decreases hepatic glucose production through the activation of gut-brain-liver axis in diabetic rats. Food Funct 9(9):4926–4935
Yujeong L, Hee Ra P, Hye Jeong C, Jaewon L (2015) Silibinin prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease via mitochondrial stabilization. J Neurosci Res 93(5):755–765
Wesołowska O, Łania‐pietrzak B, Kuzdzal M, Stanczak K, Mosiadz D (2010) Influence of silybin on biophysical properties of phospholipid bilayers1/. Acta Pharmacol Sin 28(2):296–306
Kooptiwut S, Wanchai K, Semprasert N, Srisawat C, Yenchitsomanus PT (2017) Estrogen attenuates AGTR1 expression to reduce pancreatic beta-cell death from high glucose. Sci Rep 7(1):16639. https://doi.org/10.1038/s41598-017-15237-4
Barros RP, Gustafsson JA (2011) Estrogen receptors and the metabolic network. Cell Metab 14(3):289–299. https://doi.org/10.1016/j.cmet.2011.08.005
Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F (2014) Estrogen receptors alpha (ERalpha) and beta (ERbeta): subtype-selective ligands and clinical potential. Steroids 90:13–29. https://doi.org/10.1016/j.steroids.2014.06.012
Mauvais-Jarvis F, Le May C, Tiano JP, Liu S, Kilic-Berkmen G, Kim JH (2017) The role of estrogens in pancreatic islet physiopathology. Adv Exp Med Biol 1043:385–399. https://doi.org/10.1007/978-3-319-70178-3_18
Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F (2006) Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103(24):9232–9237. https://doi.org/10.1073/pnas.0602956103
Gourdy P, Guillaume M, Fontaine C, Adlanmerini M, Montagner A, Laurell H, Lenfant F, Arnal JF (2018) Estrogen receptor subcellular localization and cardiometabolism. Mol Metab 15:56–69. https://doi.org/10.1016/j.molmet.2018.05.009
Razandi M, Pedram A, Park ST, Levin ER (2003) Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278(4):2701–2712. https://doi.org/10.1074/jbc.M205692200
Kelly MJ, Levin ER (2001) Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab 12(4):152–156
Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 81703528).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Y., Yang, J., Liu, W. et al. Attenuating effect of silibinin on palmitic acid-induced apoptosis and mitochondrial dysfunction in pancreatic β-cells is mediated by estrogen receptor alpha. Mol Cell Biochem 460, 81–92 (2019). https://doi.org/10.1007/s11010-019-03572-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03572-1