Abstract
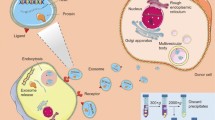
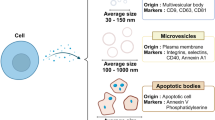
Exosomes are 40- to 100- nm cell-originated vesicles derived from endocytic compartments that are released into almost all biological fluids. Exosomes are cell-created vesicles that inherit identical phospholipid membrane, explaining a wide application of electroporation as a technique for exosomes loading with exogenous cargoes. Another way of loading exosomes with therapeutic cargo is to overexpress a certain gene in exosome-donor cells or treat cell line with drug of interest that later will be gently enveloped into vesicles based on the process of EV biogenesis. Similarly, to visualize siRNA loading into exosomes as well as the exosomal product delivery to recipient cells, we have conducted an experiment where chemical-based exosome transfection was used. In this review, we discuss different ways of extracellular vesicle loading with exogenous cargoes and their advantages/limitations as well as novel alternative techniques of substance incorporation into nanoparticles.



Similar content being viewed by others
Abbreviations
- ncRNAs:
-
Noncoding ribonucleic acid
- mRNA:
-
Messenger RNAs
- DNA:
-
Deoxyribonucleic acids
- MVBs:
-
Multivesicular bodies
- miRNAs:
-
MicroRNAs
- siRNA:
-
Small (or short) interfering RNA
- RVG:
-
Rabies viral glycoprotein
- EVs:
-
Extracellular vesicles
- MV:
-
Microvesicles
- CML:
-
Chronic myelogenous leukemia
- MAPK1:
-
Mitogen-activated protein kinase 1
- MAEC:
-
Mouse-aortic endothelial cell
- HuR:
-
Human antigen R
- EXPLORs:
-
Exosomes for protein loading via optically reversible protein–protein interactions
- CRY2:
-
Cryptochrome 2
- CIB1:
-
CRY-interacting basic-helix-loop-helix1
References
Pan BT, Johnstone R (1984) Selective externalization of the transferrin receptor by sheep reticulocytes in vitro. Response to ligands and inhibitors of endocytosis. J Biol Chem 259:9776–9782
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33:967–978
Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteom Bioinform 13:17–24. https://doi.org/10.1016/j.gpb.2015.02.001
Lotvall J, Valadi H (2007) Cell to cell signalling via exosomes through esRNA. Cell Adhes Migr 1:156–158
Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM (2010) Exosomes: fit to deliver small RNA. Commun Integr Biol 3:447–450. https://doi.org/10.4161/cib.3.5.12339
Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73:1907–1920. https://doi.org/10.1016/j.jprot.2010.06.006
Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, Shi W, He J (2014) In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomed 9:4223–4230. https://doi.org/10.2147/IJN.S64267
Yamashita T, Takahashi Y, Takakura Y (2018) Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull 41:835–842. https://doi.org/10.1248/bpb.b18-00133
Huang T, Deng CX (2019) Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. Int J Biol Sci 15:1–11. https://doi.org/10.7150/ijbs.27796
Suntres ZE, Smith MG, Momen-Heravi F, Hu J, Zhang X, Wu Y, Zhu H, Wang J, Zhou J, Kuo PW (2013) Therapeutic uses of exosomes. J Exosomes Microvesicles 1:1–8
Asadirad A, Hashemi SM, Baghaei K, Ghanbarian H, Mortaz E, Zali MR, Amani D (2019) Phenotypical and functional evaluation of dendritic cells after exosomal delivery of miRNA-155. Life Sci 219:152–162
Kyuno D, Zhao K, Bauer N, Ryschich E, Zoller M (2019) Therapeutic targeting cancer-initiating cell markers by exosome miRNA: efficacy and functional consequences exemplified for claudin7 and EpCAM. Transl Oncol 12:191–199
Pomatto MAC, Bussolati B, D’Antico S, Ghiotto S, Tetta C, Brizzi MF, Camussi G (2019) Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev 13:133–144
Faruqu FN, Xu L, Al-Jamal KT (2018) Preparation of exosomes for siRNA delivery to cancer cells. J Vis Exp 142:e58814
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546:498–503
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med 12:655–664
Ma T, Chen Y (2018) MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem cells Int 2018:3290372
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38:754–763. https://doi.org/10.1038/aps.2017.12
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, Schapira AH, Simons JP, El-Andaloussi S, Alvarez-Erviti L (2014) Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord 29:1476–1485. https://doi.org/10.1002/mds.25978
Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lasser C, Nilsson JA, Gho YS, Lotvall J (2016) RNAi delivery by exosome-mimetic nanovesicles—implications for targeting c-Myc in cancer. Biomaterials 102:231–238. https://doi.org/10.1016/j.biomaterials.2016.06.024
Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN (2016) PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology 91(241):e1–e7. https://doi.org/10.1016/j.urology.2016.01.028
Lamichhane TN, Raiker RS, Jay SM (2015) Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm 12:3650–3657. https://doi.org/10.1021/acs.molpharmaceut.5b00364
Kooijmans SA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJ, Schiffelers RM, Raemdonck K, Vader P (2013) Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release 172:229–238. https://doi.org/10.1016/j.jconrel.2013.08.014
Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci USA 112:E1433–E1442. https://doi.org/10.1073/pnas.1418401112
Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Strobel T, Breakefield XO, Saydam O (2013) Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 21:101–108. https://doi.org/10.1038/mt.2012.161
Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M (2013) Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol Ther 21:185–191. https://doi.org/10.1038/mt.2012.180
Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, Memeo L, Manno M, Raccosta S, Diana P, Cirrincione G, Giavaresi G, Monteleone F, Fontana S, De Leo G, Alessandro R (2017) Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics 7:1333–1345. https://doi.org/10.7150/thno.17092
Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N (2014) Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci 107:1–7. https://doi.org/10.1016/j.lfs.2014.04.018
Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H (2012) Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 40:e130
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV (2013) Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 11:88. https://doi.org/10.1186/1478-811X-11-88
Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3:22. https://doi.org/10.1002/0471143030.cb0322s30
Antimisiaris S, Mourtas S, Papadia K (2017) Targeted si-RNA with liposomes and exosomes (extracellular vesicles): how to unlock the potential. Int J Pharm 525:293–312. https://doi.org/10.1016/j.ijpharm.2017.01.056
Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M (2013) Sonoporation: gene transfer using ultrasound. World J Methodol 3:39–44. https://doi.org/10.5662/wjm.v3.i4.39
Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC (2017) Milk-derived exosomes for oral delivery of paclitaxel. Nanomed Nanotechnol Biol Med 13:1627–1636
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Controll Release 207:18–30
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D, Chen X, Chen Y, Yang Z, Jin L, Jiang W, Tian C, Zhou G, Zen K, Zhang J, Zhang Y, Li J, Zhang CY (2015) Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep 5:17543
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614
Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM (2016) Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng 9:315–324
O’Loughlin AJ, Mager I, de Jong OG, Varela MA, Schiffelers RM, El Andaloussi S, Wood MJA, Vader P (2017) Functional delivery of lipid-conjugated siRNA by extracellular vesicles. Mol Ther 25:1580–1587. https://doi.org/10.1016/j.ymthe.2017.03.021
Yim N, Choi C (2016) Extracellular vesicles as novel carriers for therapeutic molecules. BMB Rep 49:585–586
Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park JH, Kim D, Heo WD, Choi C (2016) Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 7:12277. https://doi.org/10.1038/ncomms12277
Acknowledgements
This work was supported by National Institute of Health Grants: HL74185, HL139047 and AR71789.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Familtseva, A., Jeremic, N. & Tyagi, S.C. Exosomes: cell-created drug delivery systems. Mol Cell Biochem 459, 1–6 (2019). https://doi.org/10.1007/s11010-019-03545-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03545-4