Abstract
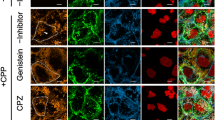
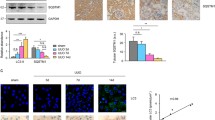
Lipotoxicity, an accumulation of intracellular lipid metabolites, has been proposed as an important pathogenic mechanism contributing to kidney dysfunction in the context of metabolic disease. Palmitic acid, a predominant lipid derivative, can cause lipoapoptosis and the release of inflammatory extracellular vesicles (EVs) in hepatocytes, but the effect of lipids on EV production in chronic kidney disease remains vaguely explored. This study was aimed to investigate whether palmitic acid would stimulate EV release from renal proximal tubular epithelial cells. Human and rat proximal tubular epithelial cells, HK-2 and NRK-52E, were incubated with 1% bovine serum albumin (BSA), BSA-conjugated palmitic acid (PA), and BSA-conjugated oleic acid (OA) for 24–48 h. The EVs released into conditioned media were isolated by ultracentrifugation and quantified by nanoparticle-tracking analysis (NTA). According to NTA, the size distribution of EVs was 30–150 nm with similar mode sizes in all experimental groups. Moreover, BSA-induced EV release was significantly enhanced in the presence of PA, whereas EV release was not altered by the addition of OA. In NRK-52E cells, PA-enhanced EV release was associated with an induction of cell apoptosis reflected by an increase in cleaved caspase-3 protein by Western blot and Annexin V positive cells analyzed by flow cytometry. Additionally, confocal microscopy confirmed the uptake of lipid-induced EVs by recipient renal proximal tubular cells. Collectively, our results indicate that PA stimulates EV release from cultured proximal tubular epithelial cells. Thus, extended characterization of lipid-induced EVs may constitute new signaling paradigms contributing to chronic kidney disease pathology.








Similar content being viewed by others
References
Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella TM, Tentori F, White S, Woodside K, Hirth RA (2016) US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States, Am J Kidney Dis67: Svii, S1–Svii, 305. https://doi.org/10.1053/j.ajkd.2015.12.014
Hager MR, Narla AD, Tannock LR (2017) Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord 18:29–40. https://doi.org/10.1007/s11154-016-9402-z
Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U (2014) Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 55:561–572. https://doi.org/10.1194/jlr.P040501
Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M (2007) Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56:2485–2493. https://doi.org/10.2337/db06-1642
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA (2018) Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 17:122. https://doi.org/10.1186/s12933-018-0762-4
Prieur X, Roszer T, Ricote M (2010) Lipotoxicity in macrophages: evidence from diseases associated with the metabolic syndrome. Biochim Biophys Acta 1801:327–337. https://doi.org/10.1016/j.bbalip.2009.09.017
Weinberg JM (2006) Lipotoxicity. Kidney Int 70:1560–1566. https://doi.org/10.1038/sj.ki.5001834
Stadler K, Goldberg IJ, Susztak K (2015) The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diabetes Rep 15:40. https://doi.org/10.1007/s11892-015-0611-8
Hua W, Huang HZ, Tan LT, Wan JM, Gui HB, Zhao L, Ruan XZ, Chen XM, Du XG (2015) CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS ONE 10:e0127507. https://doi.org/10.1371/journal.pone.0127507
Lee E, Choi J, Lee HS (2017) Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J Cell Physiol 232:3209–3217. https://doi.org/10.1002/jcp.25867
Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H, Mundel P, Jehle AW (2010) Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 299:F821–F829. https://doi.org/10.1152/ajprenal.00196.2010
Chen X, Li L, Liu X, Luo R, Liao G, Li L, Liu J, Cheng J, Lu Y, Chen Y (2018) Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci 203:291–304. https://doi.org/10.1016/j.lfs.2018.04.022
Palomer X, Pizarro-Delgado J, Barroso E, Vazquez-Carrera M (2018) Palmitic and oleic acid: the Yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol Metab 29:178–190. https://doi.org/10.1016/j.tem.2017.11.009
Cobbs A, Ballou K, Chen X, George J, Zhao X (2018) Saturated fatty acids bound to albumin enhance osteopontin expression and cleavage in renal proximal tubular cells. Int J Physiol Pathophysiol Pharmacol 10:29–38
Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ (2016) Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 126:1152–1162. https://doi.org/10.1172/JCI81129
Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ (2016) Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 150:956–967. https://doi.org/10.1053/j.gastro.2015.12.037
Lee YS, Kim SY, Ko E, Lee JH, Yi HS, Yoo YJ, Je J, Suh SJ, Jung YK, Kim JH, Seo YS, Yim HJ, Jeong WI, Yeon JE, Um SH, Byun KS (2017) Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep 7:3710. https://doi.org/10.1038/s41598-017-03389-2
Lennon R, Pons D, Sabin MA, Wei C, Shield JP, Coward RJ, Tavare JM, Mathieson PW, Saleem MA, Welsh GI (2009) Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant 24:3288–3296. https://doi.org/10.1093/ndt/gfp302
Sabin MA, Stewart CE, Crowne EC, Turner SJ, Hunt LP, Welsh GI, Grohmann MJ, Holly JM, Shield JP (2007) Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J Cell Physiol 211:244–252. https://doi.org/10.1002/jcp.20922
Mook S, Halkes CC, Bilecen S, Cabezas MC (2004) In vivo regulation of plasma free fatty acids in insulin resistance. Metabolism 53:1197–1201
Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L (2010) Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol 5:2373–2379. https://doi.org/10.2215/CJN.08160910
Nakamura K, Sawada K, Kinose Y, Yoshimura A, Toda A, Nakatsuka E, Hashimoto K, Mabuchi S, Morishige KI, Kurachi H, Lengyel E, Kimura T (2017) Exosomes promote ovarian cancer cell invasion through transfer of CD44 to peritoneal mesothelial cells. Mol Cancer Res 15:78–92. https://doi.org/10.1158/1541-7786.MCR-16-0191
Huang MB, Gonzalez RR, Lillard J, Bond VC (2017) Secretion modification region-derived peptide blocks exosome release and mediates cell cycle arrest in breast cancer cells. Oncotarget 8:11302–11315. https://doi.org/10.18632/oncotarget.14513
Baumann JM, Kokabee L, Wang X, Sun Y, Wong J, Conklin DS (2015) Metabolic assays for detection of neutral fat stores. Bio Protoc 5:12
Cohen BC, Shamay A, Argov-Argaman N (2015) Regulation of lipid droplet size in mammary epithelial cells by remodeling of membrane lipid composition-a potential mechanism. PLoS ONE 10:e0121645. https://doi.org/10.1371/journal.pone.0121645
Akoumi A, Haffar T, Mousterji M, Kiss RS, Bousette N (2017) Palmitate mediated diacylglycerol accumulation causes endoplasmic reticulum stress, Plin2 degradation, and cell death in H9C2 cardiomyoblasts. Exp Cell Res 354:85–94. https://doi.org/10.1016/j.yexcr.2017.03.032
Plotz T, Hartmann M, Lenzen S, Elsner M (2016) The role of lipid droplet formation in the protection of unsaturated fatty acids against palmitic acid induced lipotoxicity to rat insulin-producing cells. Nutr Metab (Lond) 13:16. https://doi.org/10.1186/s12986-016-0076-z
Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE (2006) Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 47:2726–2737. https://doi.org/10.1194/jlr.M600299-JLR200
Katsoulieris E, Mabley JG, Samai M, Sharpe MA, Green IC, Chatterjee PK (2010) Lipotoxicity in renal proximal tubular cells: relationship between endoplasmic reticulum stress and oxidative stress pathways. Free Radic Biol Med 48:1654–1662. https://doi.org/10.1016/j.freeradbiomed.2010.03.021
Pomatto MAC, Gai C, Bussolati B, Camussi G (2017) Extracellular vesicles in renal pathophysiology. Front Mol Biosci 4:37. https://doi.org/10.3389/fmolb.2017.00037
Ban LA, Shackel NA, McLennan SV (2016) Extracellular vesicles: a new frontier in biomarker discovery for non-alcoholic fatty liver disease. Int J Mol Sci 17:376. https://doi.org/10.3390/ijms17030376
Harp D, Driss A, Mehrabi S, Chowdhury I, Xu W, Liu D, Garcia-Barrio M, Taylor RN, Gold B, Jefferson S, Sidell N, Thompson W (2016) Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res 365:187–196. https://doi.org/10.1007/s00441-016-2358-1
Yamamoto CM, Murakami T, Oakes ML, Mitsuhashi M, Kelly C, Henry RR, Sharma K (2018) Uromodulin mRNA from urinary extracellular vesicles correlate to kidney function decline in type 2 diabetes mellitus. Am J Nephrol 47:283–291. https://doi.org/10.1159/000489129
Dominguez JM, Dominguez JH, Xie D, Kelly KJ (2018) Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PLoS ONE 13:e0202550. https://doi.org/10.1371/journal.pone.0202550
Gutierrez-Vazquez C, Villarroya-Beltri C, Mittelbrunn M, Sanchez-Madrid F (2013) Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev 251:125–142. https://doi.org/10.1111/imr.12013
De S, Kuwahara S, Hosojima M, Ishikawa T, Kaseda R, Sarkar P, Yoshioka Y, Kabasawa H, Iida T, Goto S, Toba K, Higuchi Y, Suzuki Y, Hara M, Kurosawa H, Narita I, Hirayama Y, Ochiya T, Saito A (2017) Exocytosis-mediated urinary full-length megalin excretion is linked with the pathogenesis of diabetic nephropathy. Diabetes 66:1391–1404. https://doi.org/10.2337/db16-1031
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL, Zhou LT, Wang B, Zhang JD, Crowley SD, Liu BC (2018) Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol 29:919–935. https://doi.org/10.1681/ASN.2017050523
Musso G, Cassader M, Cohney S, De MF, Pinach S, Saba F, Gambino R (2016) Fatty liver and chronic kidney disease: novel mechanistic insights and therapeutic opportunities. Diabetes Care 39:1830–1845. https://doi.org/10.2337/dc15-1182
Caruso S, Poon IKH (2018) Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol 9:1486. https://doi.org/10.3389/fimmu.2018.01486
Lynch C, Panagopoulou M, Gregory CD (2017) Extracellular vesicles arising from apoptotic cells in tumors: roles in cancer pathogenesis and potential clinical applications. Front Immunol 8:1174. https://doi.org/10.3389/fimmu.2017.01174
Atkin-Smith GK, Poon IKH (2017) Disassembly of the dying: mechanisms and functions. Trends Cell Biol 27:151–162. https://doi.org/10.1016/j.tcb.2016.08.011
Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z (2016) Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311:F844–F851. https://doi.org/10.1152/ajprenal.00429.2016
Lu CC, Ma KL, Ruan XZ, Liu BC (2017) The emerging roles of microparticles in diabetic nephropathy. Int J Biol Sci 13:1118–1125. https://doi.org/10.7150/ijbs.21140
Acknowledgements
This work was supported by the NIH SC1DK112151, NIH 5T32HL103104-07, NIH/NCRR/RCMI 8G12MD007602 and 8U54MD007588.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cobbs, A., Chen, X., Zhang, Y. et al. Saturated fatty acid stimulates production of extracellular vesicles by renal tubular epithelial cells. Mol Cell Biochem 458, 113–124 (2019). https://doi.org/10.1007/s11010-019-03535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03535-6