Abstract
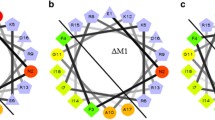
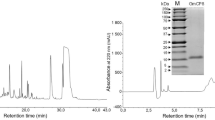
Apolipophorin III (apoLp-III) is an insect apolipoprotein that is predominantly present in a lipid-free state in the hemolymph. ApoLp-III from Galleria mellonella is able to interact with membrane components of Gram-negative bacteria, as part of an innate immune response to infection. The protein also exists in a lipoprotein-associated state when large amounts of lipids are mobilized. Therefore, lipid-bound apoLp-III was generated to analyze the binding interaction with lipopolysaccharides and phosphatidylglycerol, both abundantly present in membranes of Gram-negative bacteria. G. mellonella apoLp-III was lipidated with palmitoyl-2-oleoyl-glycero-3-phosphocholine to form lipid-protein complexes. The particle shape was discoidal with a 16.4 nm diameter, a molecular mass of 460 kDa, and contained 4 apoLp-III molecules. These discoidal lipoproteins were used to compare the lipopolysaccharide and phosphatidylglycerol binding activity with lipid-free apoLp-III. Lipopolysaccharide binding interaction was analyzed by non-denaturing PAGE, showing reduced ability of the lipid-bound protein to form lipopolysaccharide-protein complexes and to disaggregate lipopolysaccharide micelles. The apoLp-III-induced release of calcein from phosphatidylglycerol vesicles was decreased approximately fivefold when the protein was in the lipid-bound form, indicating reduced binding interaction with the phosphatidylglycerol membrane surface. These results show that when apoLp-III adopts a lipid-bound conformation, it is markedly less effective in interacting with lipopolysaccharides and phosphatidylglycerol vesicles. Thus, in order to be an effective antimicrobial protein, apoLp-III needs to be in a lipid-free state.






Similar content being viewed by others
References
Zdybicka-Barabas A, Cytryńska M (2013) Apolipophorins and insects immune response. ISJ 10:58–68
Weise C, Franke P, Kopácek P, Wiesner A (1998) Primary structure of apolipophorin-III from the greater wax moth, Galleria mellonella. J Protein Chem 17:633–641
Wang J, Sykes BD, Ryan RO (2002) Structural basis for the conformational adaptability of apolipophorin III, a helix-bundle exchangeable apolipoprotein. Proc Natl Acad Sci USA 99:1188–1193
Breiter DR, Kanost MR, Benning MM, Wesenberg G, Law JH, Wells MA, Rayment I, Holden HM (1991) Molecular structure of an apolipoprotein determined at 2.5 Å resolution. Biochemistry 30:603–608
Hård K, Van Doorn JM, Thomas-Oates JE, Kamerling JP, Van der Horst DJ (1993) Structure of the Asn-linked oligosaccharides of apolipophorin III from the insect Locusta migratoria. Carbohydrate-linked 2-aminoethylphosphonate as a constituent of a glycoprotein. Biochemistry 32:766–775
Weers PMM, Ryan RO (2006) Apolipophorin III: role model apolipoprotein. Insect Biochem Mol Biol 36:231–240
Weers PMM, Ryan RO (2003) Apolipophorin III: a lipid-triggered molecular switch. Insect Biochem Mol Biol 33:1249–1260
Van der Horst DJ, van Hoof D, van Marrewijk WJ, Rodenburg KW (2002) Alternative lipid mobilization: the insect shuttle system. Mol Cell Biochem 239:113–119
Wientzek M, Kay CM, Oikawa K, Ryan RO (1994) Binding of insect apolipophorin III to dimyristoylphosphatidylcholine vesicles. Evidence for a conformational change. J Biol Chem 269:4605–4612
Van der Horst DJ, Rodenburg KW (2010) Locust flight activity as a model for hormonal regulation of lipid mobilization and transport. J Insect Physiol 56:844–853
Kawooya JK, van der Horst DJ, van Heusden MC, Brigot BL, van Antwerpen R, Law JH (1991) Lipophorin structure analyzed by in vitro treatment with lipases. J Lipid Res 32:1781–1788
Weers PMM, Cabrera J, Abdullahi WE, Hsu T-C (2005) Role of buried polar residues in helix bundle stability and lipid binding of apolipophorin III: destabilization by threonine 31. Biochemistry 44:8810–8816
Thistle J, Martinon D, Weers PMM (2015) Helix 1 tryptophan variants in Galleria mellonella apolipophorin III. Chem Phys Lipids 193:18–23
Wiesner A, Losen S, Kopáček P, Weise C, Götz P (1997) Isolated apolipophorin III from Galleria mellonella stimulates the immune reactions of this insect. J Insect Physiol 43:383–391
Niere M, Meisslitzer C, Dettloff M, Weise C, Ziegler M, Wiesner A (1999) Insect immune activation by recombinant Galleria mellonella apolipophorin III. Biochim Biophys Acta 1433:16–26
Halwani AE, Dunphy GB (1999) Apolipophorin-III in Galleria mellonella potentiates hemolymph lytic activity. Dev Comp Immunol 23:563–570
Halwani AE, Niven DF, Dunphy GB (2000) Apolipophorin-III and the interactions of lipoteichoic acids with the immediate immune responses of Galleria mellonella. J Invertebr Pathol 76:233–241
Zdybicka-Barabas A, Palusińska-Szysz M, Gruszecki WI, Mak P, Cytryńska M (2014) Galleria mellonella apolipophorin III—an apolipoprotein with anti-Legionella pneumophila activity. Biochim Biophys Acta 1838:2689–2697
Pratt CC, Weers PMM (2004) Lipopolysaccharide binding of an exchangeable apolipoprotein, apolipophorin III, from Galleria mellonella. Biol Chem 385:1113–1119
Iimura Y, Ishikawa H, Yamamoto K, Sehnal F (1998) Hemagglutinating properties of apolipophorin III from the hemolymph of Galleria mellonella larvae. Arch Insect Biochem Physiol 38:119–125
Zdybicka-Barabas A, Cytryńska M (2011) Involvement of apolipophorin III in antibacterial defense of Galleria mellonella larvae. Comp Biochem Physiol B: Biochem Mol Biol 158:90–98
Dettloff M, Kaiser B, Wiesner A (2001) Localization of injected apolipophorin III in vivo—new insights into the immune activation process directed by this protein. J Insect Physiol 47:789–797
Dettloff M, Wittwer D, Weise C, Wiesner A (2001) Lipophorin of lower density is formed during immune responses in the lepidopteran insect Galleria mellonella. Cell Tissue Res 306:449–458
Niere M, Dettloff M, Maier T, Ziegler M, Wiesner A (2001) Insect immune activation by apolipophorin III is correlated with the lipid-binding properties of this protein. Biochemistry 40:11502–11508
Leon LJ, Pratt CC, Vasquez LJ, Weers PMM (2006) Tyrosine fluorescence analysis of apolipophorin III-lipopolysaccharide interaction. Arch Biochem Biophys 452:38–45
Leon LJ, Idangodage H, Wan LC-P, Weers PMM (2006) Apolipophorin III: lipopolysaccharide binding requires helix bundle opening. Biochem Biophys Res Commun 348:1322–1328
Oztug M, Martinon D, Weers PMM (2012) Characterization of the apoLp-III/LPS complex: insight into the mode of binding interaction. Biochemistry 51:6220–6227
Lee CH, Tsai CM (1999) Quantification of bacterial lipopolysaccharides by the purpald assay: measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal Biochem 267:161–168
Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118
Liu H, Scraba DG, Ryan RO (1993) Prevention of phospholipase-C induced aggregation of low density lipoprotein by amphipathic apolipoproteins. FEBS Lett 316:27–33
Beck WHJ, Adams CP, Biglang-awa IM, Patel AB, Vincent H, Haas-Stapleton EJ, Weers PMM (2013) Apolipoprotein A–I binding to anionic vesicles and lipopolysaccharides: role for lysine residues in antimicrobial properties. Biochim Biophys Acta (Biomembranes) 1828:1503–1510
Dettloff M, Weers PMM, Niere M, Kay CM, Ryan RO, Wiesner A (2001) An N-terminal three-helix fragment of the exchangeable insect apolipoprotein apolipophorin III conserves the lipid binding properties of wild type protein. Biochemistry 40:3150–3157
Sahoo D, Weers PMM, Ryan RO, Narayanaswami V (2002) Lipid-triggered conformational switch of apolipophorin III helix bundle to an extended helix organization. J Mol Biol 321:201–214
Garda HA, Arrese EL, Soulages JL (2002) Structure of apolipophorin-III in discoidal lipoproteins. Interhelical distances in the lipid-bound state and conformational change upon binding to lipid. J Biol Chem 277:19773–19782
Mullen L, Goldsworthy G (2003) Changes in lipophorins are related to the activation of phenoloxidase in the haemolymph of Locusta migratoria in response to injection of immunogens. Insect Biochem Mol Biol 33:661–670
Mullen LM, Lightfoot ME, Goldsworthy GJ (2004) Induced hyperlipaemia and immune challenge in locusts. J Insect Physiol 50:409–417
Liu H, Malhotra V, Ryan RO (1991) Displacement of apolipophorin III from the surface of low density lipophorin by human apolipoprotein A-I. Biochem Biophys Res Commun 179:734–740
Goldsworthy GJ, Opoku-Ware K, Mullen LM (2005) Adipokinetic hormone and the immune responses of locusts to infection. Ann N Y Acad Sci 1040:106–113
Adamo SA, Roberts JL, Easy RH, Ross NW (2008) Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J Exp Biol 211:531–538
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number GM089564. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This study was funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number GM089564.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wijeratne, T.U., Weers, P.M.M. Lipid-bound apoLp-III is less effective in binding to lipopolysaccharides and phosphatidylglycerol vesicles compared to the lipid-free protein. Mol Cell Biochem 458, 61–70 (2019). https://doi.org/10.1007/s11010-019-03530-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03530-x