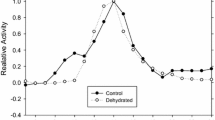
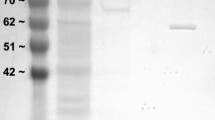
Abstract
Carbamoyl phosphate synthetase I (CPS1) represents an important regulatory enzyme of the urea cycle that mediates the ATP-driven reaction ligating ammonium, carbonate, and phosphate to form carbamoyl phosphate. The freeze-tolerant wood frog (Rana sylvatica or Lithobates sylvaticus) accumulates high concentrations of urea during bouts of freezing to detoxify any ammonia generated and to contribute as a cryoprotectant thereby helping to avoid freeze damage to cells. Purification of CPS1 to homogeneity from wood frog liver was performed in control and frozen wood frogs by a three-step chromatographic process. The affinity of CPS1 for its three substrates was tested in the purified control and freeze-exposed enzyme under a variety of conditions including the presence and absence of the natural cryoprotectants urea and glucose. The results demonstrated that affinity for ammonium was higher in the freeze-exposed CPS1 (1.26-fold) and that with the addition of 400 mM glucose it displayed higher affinity for ATP (1.30-fold) and the obligate activator N-acetylglutamate (1.24-fold). Denaturation studies demonstrated the freeze-exposed enzyme was less thermally stable than the control with an unfolding temperature approximately 1.5 °C lower (52.9 °C for frozen and 54.4 °C for control). The control form of CPS1 had a significantly higher degree of glutarylated lysine residues (1.42-fold increase) relative to the frozen. The results suggest that CPS1 activation and maintenance of urea cycle activity despite the hypometabolic conditions associated with freezing are important aspects in the metabolic survival strategies of the wood frog.




Similar content being viewed by others
References
Regosin JV, Windmiller BS, Reed JM (2003) Terrestrial habitat use and winter densities of the wood frog (Rana sylvatica). J Herpetol 37:390–394
Layne JR, Lee RE (1987) Freeze tolerance and the dynamics of ice formation in wood frogs (Rana sylvatica) from southern Ohio. Can J Zool 65:2062–2065. https://doi.org/10.1139/z87-315
Storey KB, Storey JM (1988) Freeze tolerance in animals. Physiol Rev 68:27–84
Costanzo JP, Lee RE, Lortz PH (1993) Glucose concentration regulates freeze tolerance in the wood frog Rana sylvatica. J Exp Biol 181:245–255
Dieni CA, Bouffard MC, Storey KB (2012) Glycogen synthase kinase-3: cryoprotection and glycogen metabolism in the freeze-tolerant wood frog. J Exp Biol 215:543–551. https://doi.org/10.1242/jeb.065961
Russell EL, Storey KB (1995) Glycogen-synthetase and the control of cryoprotectant clearance after thawing in the freeze-tolerant wood frog. Cryo-Letters 16:263–266
Do Amaral MCF, Lee RE, Costanzo JP (2013) Enzymatic regulation of glycogenolysis in a subarctic population of the wood frog: implications for extreme freeze tolerance. PLoS ONE 8:1–10. https://doi.org/10.1371/journal.pone.0079169
Storey KB, Storey JM (2017) Molecular physiology of freeze tolerance in vertebrates. Physiol Rev 97:623–665
Costanzo JP, Reynolds AM, do Amaral MCF et al (2015) Cryoprotectants and extreme freeze tolerance in a subarctic population of the wood frog. PLoS ONE 10:e0117234. https://doi.org/10.1371/journal.pone.0117234
Costanzo JP, Lee RE (2008) Urea loading enhances freezing survival and postfreeze recovery in a terrestrially hibernating frog. J Exp Biol 211:2969–2975. https://doi.org/10.1242/jeb.019695
Jørgensen CB (1997) Urea and amphibian water economy. Comp Biochem Physiol 117:161–170
Costanzo JP, Lee RE (2005) Cryoprotection by urea in a terrestrially hibernating frog. J Exp Biol 208:4079–4089. https://doi.org/10.1242/jeb.01859
Grundy JE, Storey KB (1994) Urea and salt effects on enzymes from estivating and non-estivating amphibians. Mol Cell Biochem 131:9–17. https://doi.org/10.1007/BF01075719
Bakhach J (2009) The cryopreservation of composite tissues: Principles and recent advancement on cryopreservation of different type of tissues. Organogenesis 5:119–126. https://doi.org/10.4161/org.5.3.9583
Costanzo JP, do Amaral MCF, Rosendale AJ, Lee RE (2013) Hibernation physiology, freezing adaptation and extreme freeze tolerance in a northern population of the wood frog. J Exp Biol 216:3461–3473. https://doi.org/10.1242/jeb.089342
De Cima S, Polo LM, Díez-Fernández C et al (2015) Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis. Sci Rep 5:16950. https://doi.org/10.1038/srep16950
Tuchman M, Holzknecht RA (1990) N-acetylglutamate content in liver and gut of normal and fasted mice, normal human livers, and livers of individuals with carbamyl phosphate synthetase or ornithine transcarbamylase deficiency. Pediatr Res 27:408–412. https://doi.org/10.1203/00006450-199004000-00020
Ogura M, Nakamura Y, Tanaka D et al (2010) Overexpression of SIRT5 confirms its involvement in deacetylation and activation of carbamoyl phosphate synthetase 1. Biochem Biophys Res Commun 393:73–78. https://doi.org/10.1016/j.bbrc.2010.01.081
Guan KL, Xiong Y (2011) Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci 36:108–116
Du J, Zhou Y, Su X et al (2011) Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334:806–809. https://doi.org/10.1126/science.1207861
Lin H, Su X, He B (2012) Protein lysine acylation and cysteine succination by intermediates of energy metabolism. ACS Chem Biol 7:947–960
Wright P, Anderson P, Weng L et al (2004) The crab-eating frog, Rana cancrivora, up-regulates hepatic carbamoyl phosphate synthetase I activity and tissue osmolyte levels in response to increased salinity. J Exp Zool Part A 301:559–568. https://doi.org/10.1002/jez.a.54
Schiller TM, Costanzo JP, Lee RE (2008) Urea production capacity in the wood frog (Rana sylvatica) varies with season and experimentally induced hyperuremia. J Exp Zool Part A 309A:484–493
Kiss AJ, Muir TJ, Lee RE, Costanzo JP (2011) Seasonal variation in the hepatoproteome of the dehydration and freeze-tolerant wood frog, Rana sylvatica. Int J Mol Sci 12:8406–8414. https://doi.org/10.3390/ijms12128406
Green SR, Storey KB (2017) Regulation of glutamate dehydrogenase (GDH) in response to whole body freezing in wood frog liver linked to differential acetylation and ADP-ribosylation. Arch Biochem Biophys 636:90–99. https://doi.org/10.1016/j.abb.2017.10.010
Dieni CA, Storey KB (2014) Protein kinase C in the wood frog, Rana sylvatica: reassessing the tissue-specific regulation of PKC isozymes during freezing. PeerJ 2:e558. https://doi.org/10.7717/peerj.558
Childers CL, Storey KB (2016) Post-translational regulation of hexokinase function and protein stability in the aestivating frog Xenopus laevis. Protein J 35:61–71. https://doi.org/10.1007/s10930-016-9647-0
Gromova I, Celis JE (2006) Protein detection in gels by silver staining: a procedure compatible with mass spectrometry. In: Celis JE (ed) Cell biology: a laboratory handbook. Elsevier Science, New York, pp 219–223
Kamau Machua S, Mukuria JC, Ngure RM, Gitu PM (2014) Modulation of partially purified rat liver mitochondrial carbamoyl phosphate synthetase I using two glutamic acid analogues. Unique Res J Biochem Biotechnol 1:1–10
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Green SR, Storey KB (2016) Regulation of crayfish, Orconectes virilis, tail muscle lactate dehydrogenase (LDH) in response to anoxic conditions is associated with alterations in phosphorylation patterns. Comp Biochem Physiol Part B 202:67–74. https://doi.org/10.1016/j.cbpb.2016.08.004
Brooks SP (1992) A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 13:906–911
Brooks SP (1994) A program for analyzing enzyme rate data obtained from a microplate reader. Biotechniques 17:1154–1161
Zhang J, Storey KB (2016) RBioplot: an easy-to-use R pipeline for automated statistical analysis and data visualization in molecular biology and biochemistry. PeerJ 4:e2436. https://doi.org/10.7717/peerj.2436
Tan M, Peng C, Anderson KA et al (2014) Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab 19:605–617. https://doi.org/10.1016/j.cmet.2014.03.014
Harmel R, Fiedler D (2018) Features and regulation of non-enzymatic post-translational modifications. Nat Chem Biol 14:244–252
Strauss KA, Brumbaugh J, Duffy A et al (2011) Safety, efficacy and physiological actions of a lysine-free, arginine-rich formula to treat glutaryl-CoA dehydrogenase deficiency: Focus on cerebral amino acid influx. Mol Genet Metab 104:93–106. https://doi.org/10.1016/j.ymgme.2011.07.003
Storey KB, Storey JM (1986) Freeze tolerant frogs: cryoprotectants and tissue metabolism during freeze–thaw cycles. Can J Zool 64:49–56
Wiebler JM, Kohl KD, Lee RE, Costanzo JP (2018) Urea hydrolysis by gut bacteria in a hibernating frog: evidence for urea-nitrogen recycling in Amphibia. Proc R Soc 285:20180241. https://doi.org/10.1098/rspb.2018.0241
Katzenback BA, Dawson NJ, Storey KB (2014) Purification and characterization of a urea sensitive lactate dehydrogenase from the liver of the African clawed frog, Xenopus laevis. J Comp Physiol B 184:601–611. https://doi.org/10.1007/s00360-014-0824-1
Muir TJ, Costanzo JP, Lee RE (2008) Metabolic depression induced by urea in organs of the wood frog, Rana sylvatica: effects of season and temperature. J Exp Zool Part A 309:111–116. https://doi.org/10.1002/jez.436
García-España A, Alonso E, Rubio V (1991) Influence of anions on the activation of carbamoyl phosphate synthetase (ammonia) by acetylglutamate: Implications for the activation of the enzyme in the mitochondria. Arch Biochem Biophys 288:414–420. https://doi.org/10.1016/0003-9861(91)90214-4
Solaini G, Baracca A, Lenaz G, Sgarbi G (2010) Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 1797:1171–1177. https://doi.org/10.1016/j.bbabio.2010.02.011
Struvay C, Feller G (2012) Optimization to low temperature activity in psychrophilic enzymes. Int J Mol Sci 13:11643–11665. https://doi.org/10.3390/ijms130911643
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43. https://doi.org/10.1128/MMBR.65.1.1-43.2001
Acknowledgements
The authors would like to thank J.M. Storey and C.L. Childers for their help in editing the manuscript. The research was funded by a Discovery Grant (Number: 6793) through the Natural Sciences and Engineering Research Council of Canada (NSERC). K.B. Storey currently holds the Canada Research Chair in Molecular Physiology and S.R. Green was a recipient of a Queen Elizabeth II Graduate Scholarship in Science and Technology during the time of the study.
Funding
The research was funded by a Discovery Grant (Number: 6793) through the Natural Sciences and Engineering Research Council of Canada (NSERC). K.B. Storey currently holds the Canada Research Chair in Molecular Physiology and S.R. Green was a recipient of a Queen Elizabeth II Graduate Scholarship in Science and Technology during the time of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Green, S.R., Storey, K.B. Purification of carbamoyl phosphate synthetase 1 (CPS1) from wood frog (Rana sylvatica) liver and its regulation in response to ice-nucleation and subsequent whole-body freezing. Mol Cell Biochem 455, 29–39 (2019). https://doi.org/10.1007/s11010-018-3468-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3468-8