Abstract
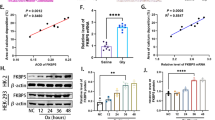
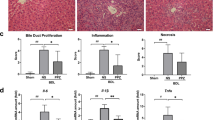
Hepatolithiasis is commonly encountered in Southeastern and Eastern Asian countries, but the pathogenesis mechanism of stone formation is still not well understood. Now, the role of endogenous β-glucuronidase in pigment stones formation is being gradually recognized. In this study, the mechanism of increased expression and secretion of endogenous β-glucuronidase during hepatolithiasis formation was investigated. We assessed the endogenous β-glucuronidase, c-myc, p-p65, and p-PKC expression in liver specimens with hepatolithiasis by immunohistochemical staining, and found that compared with that in normal liver samples, the expression of endogenous β-glucuronidase, c-myc, p-p65, and p-PKC in liver specimens with hepatolithiasis significantly increased, and their expressions were positively correlated with each other. Lipopolysaccharide (LPS) induced increased expression of endogenous β-glucuronidase and c-myc in hepatocytes and intrahepatic biliary epithelial cells in a dose- and time-dependent manner, and endogenous β-glucuronidase secretion increased, correspondingly. C-myc siRNA transfection effectively inhibited the LPS-induced expression of endogenous β-glucuronidase. Furthermore, NF-κB inhibitor pyrrolidine dithiocarbamate or PKC inhibitor chelerythrine could effectively inhibit the LPS-induced expression of c-myc and endogenous β-glucuronidase, and the expression of p-p65 was also partly inhibited by chelerythrine. Our clinical observations and experimental data indicate that LPS could induce the increased expression and secretion of endogenous β-glucuronidase via a signaling cascade of PKC/NF-κB/c-myc in hepatocytes and intrahepatic biliary epithelial cells, and endogenous β-glucuronidase might play a possible role in the formation of hepatolithiasis.






Similar content being viewed by others
Abbreviations
- LPS:
-
Lipopolysaccharide
- NF-κB:
-
Nuclear factor kappa B
- PKC:
-
Protein kinase C
- PI3K:
-
Phosphoinositide-3-kinase
- IKK:
-
IκB-α kinase
References
Dey B, Kaushal G, Jacob SE, Barwad A, Pottakkat B (2016) Pathogenesis and management of hepatolithiasis: a report of two cases. J Clin Diagn Res 10:PD11–P13
Tazuma S, Unno M, Igarashi Y, Inui K, Uchiyama K, Kai M, Tsuyuguchi T, Maguchi H, Mori T, Yamaguchi K, Ryozawa S, Nimura Y, Fujita N, Kubota K, Shoda J, Tabata M, Mine T, Sugano K, Watanabe M, Shimosegawa T (2017) Evidence-based clinical practice guidelines for cholelithiasis 2016. J Gastroenterol 52:276–300
Tazuma S (2006) Gallstone disease: epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol 20:1075–1083
Tsui WM, Chan YK, Wong CT, Lo YF, Yeung YW, Lee YW (2011) Hepatolithiasis and the syndrome of recurrent pyogenic cholangitis: clinical, radiologic, and pathologic features. Semin Liver Dis 31:33–48
Maki T (1966) Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg 164:90–100
Ho KJ, Hsu SC, Chen JS, Ho LH (1986) Human biliary beta-glucuronidase: correlation of its activity with deconjugation of bilirubin in the bile. Eur J Clin Invest 16:361–367
Ho KJ, Lin XZ, Yu SC, Chen JS, Wu CZ (1995) Cholelithiasis in Taiwan: gallstone characteristics, surgical incidence, bile lipid composition, and role of beta-glucuronidase. Dig Dis Sci 40:1963–1973
Li N, Xiao L, Cheng S, Xiao B, Cheng W, Li Q (1990) [The changes of beta-glucuronidase in rabbit model with calcium bilirubinate stone]. Hua Xi Yi Ke Da Xue Xue Bao 21:185–187 (Article in Chinese)
Zhang XB, Cui NQ, Li DH (2007) [Effect of clearing heat and removing dampness method on formation of pigment gallstones in rabbits]. Zhongguo Zhong Xi Yi Jie He Za Zhi 27:241–243 (Article in Chinese).
Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC (2007) HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11:335–347
Gordan JD, Thompson CB, Simon MC (2007) HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12:108–113
Chan KL, Guan XY, Ng IO (2004) High-throughput tissue microarray analysis of c-myc activation in chronic liver diseases and hepatocellular carcinoma. Hum Pathol 35:1324–1331
Li FY, Cheng NS, Cheng JQ, Mao H, Jiang LS, Li N, He S (2009) Treatment of chronic proliferative cholangitis with c-myc shRNA. World J Gastroenterol 15:95–101
Ganguly N, Giang PH, Basu SK, Mir FA, Siddiqui I, Sharma P (2007) Mycobacterium tuberculosis 6-kDa early secreted antigenic target (ESAT-6) protein downregulates lipopolysaccharide induced c-myc expression by modulating the extracellular signal regulated kinases 1/2. BMC Immunol 8:24
Grumont RJ, Strasser A, Gerondakis S (2002) B cell growth is controlled by phosphatidylinosotol 3-kinase-dependent induction of Rel/NF-kappaB regulated c-myc transcription. Mol Cell 10:1283–1294
Mishra S, Vinayak M (2013) Ellagic acid checks lymphoma promotion via regulation of PKC signaling pathway. Mol Biol Rep 40:1417–1428
Su Y, Wu SD, Jin JZ, Zhang ZH, Fan Y (2006) Role of intestinal barrier in pathogenesis of pigment gallstone in a guinea pig model. Hepatobiliary Pancreat Dis Int 5:443–448
Vítek L, Carey MC (2012) New pathophysiological concepts underlying pathogenesis of pigment gallstones. Clin Res Hepatol Gastroenterol 36:122–129
Stewart L, Grifiss JM, Jarvis GA, Way LW (2006) Biliary bacterial factors determine the path of gallstone formation. Am J Surg 192:598–603
Ho KJ (1985) Human beta-glucuronidase. Studies on the effects of pH and bile acids in regard to its role in the pathogenesis of cholelithiasis. Biochim Biophys Acta 827:197–206
Chen CY, Shiesh SC, Tsao HC, Lin XZ (2000) Human biliary beta-glucuronidase activity before and after relief of bile duct obstruction: is it the major role in the formation of pigment gallstones. J Gastroenterol Hepatol 15:1071–1075
Sethi G, Ahn KS, Aggarwal BB (2008) Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res 6:1059–1070
Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, Abraham E (2005) Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal 17:385–394
Lee M, Jeon YJ (2001) Paclitaxel-induced immune suppression is associated with NF-kappaB activation via conventional PKC isotypes in lipopolysaccharide-stimulated 70Z/3 pre-B lymphocyte tumor cells. Mol Pharmacol 59:248–253
Zhou X, Yang W, Li J (2006) Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-kappaB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-alpha production in lipopolysaccharide-stimulated rat peritoneal macrophages. J Biol Chem 281:31337–31347
Zeng KW, Li J, Dong X, Wang YH, Ma ZZ, Jiang Y, Jin HW, Tu PF (2013) Anti-neuroinflammatory efficacy of the aldose reductase inhibitor FMHM via phospholipase C/protein kinase C-dependent NF-κB and MAPK pathways. Toxicol Appl Pharmacol 273:159–171
Baud V, Karin M (2009) Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 8:33–40
Bourgarel-Rey V, Vallee S, Rimet O, Champion S, Braguer D, Desobry A, Briand C, Barra Y (2001) Involvement of nuclear factor kappaB in c-Myc induction by tubulin polymerization inhibitors. Mol Pharmacol 59:1165–1170
Duyao MP, Buckler AJ, Sonenshein GE (1990) Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proc Natl Acad Sci USA 87:4727–4731
Duyao MP, Kessler DJ, Spicer DB, Sonenshein GE (1990) Binding of NF-KB-like factors to regulatory sequences of the c-myc gene. Curr Top Microbiol Immunol 166:211–220
Kessler DJ, Duyao MP, Spicer DB, Sonenshein GE (1992) NF-kappa B-like factors mediate interleukin 1 induction of c-myc gene transcription in fibroblasts. J Exp Med 176:787–792
Byun E, Park B, Lim JW, Kim H (2016) Activation of NF-κB and AP-1 mediates hyperproliferation by inducing β-catenin and c-Myc in helicobacter pylori-infected gastric epithelial cells. Yonsei Med J 57:647–651
Ji Y, Wang Z, Li Z, Zhang A, Jin Y, Chen H, Le X (2016) Angiotensin II enhances proliferation and inflammation through AT1/PKC/NF-κB signaling pathway in hepatocellular carcinoma cells. Cell Physiol Biochem 39:13–32
Kang YK, Putluri N, Maity S, Tsimelzon A, Ilkayeva O, Mo Q, Lonard D, Michailidis G, Sreekumar A, Newgard CB, Wang M, Tsai SY, Tsai MJ, O’Malley BW (2015) CAPER is vital for energy and redox homeostasis by integrating glucose-induced mitochondrial functions via ERR-α-Gabpa and stress-induced adaptive responses via NF-κB-cMYC. PLoS Genet 11:e1005116
Lu Y, Zhu M, Chen W, Yin L, Zhu J, Chen N, Chen W (2014) Tetramethylpyrazine improves oxazolone-induced colitis by inhibiting the NF-κB pathway. Clin Invest Med 37:E1–E9
Singh AR, Peirce SK, Joshi S, Durden DL (2014) PTEN and PI-3 kinase inhibitors control LPS signaling and the lymphoproliferative response in the CD19+ B cell compartment. Exp Cell Res 327:78–90
Acknowledgements
This work was funded by National Natural Science Foundation of China (81400659).
Author information
Authors and Affiliations
Contributions
YD and DQ performed experiments; YD and TY analyzed data, interpreted results of experiments, and prepared figures; YD, DC, and WS designed the overall study; YD drafted manuscript; DC and WS approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical approval
The study was reviewed and approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University (reference number: 2012PS34K) and written informed consent was obtained from all patients, and performed in accordance with the principles outlined in the Declaration of Helsinki.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, D., Dong, Q., Tian, Y. et al. Lipopolysaccharide stimulates endogenous β-glucuronidase via PKC/NF-κB/c-myc signaling cascade: a possible factor in hepatolithiasis formation. Mol Cell Biochem 444, 93–102 (2018). https://doi.org/10.1007/s11010-017-3234-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3234-3