Abstract
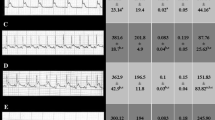
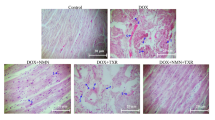
Doxorubicin (DOX), an anthracycline-based antibiotic, is regularly used in the management of carcinomas, and haematological malignancies have been downplayed in chemotherapy because of its ability to induce dilated cardiomyopathy (DCM). Dexrazoxane is approved to combat the cardiotoxicity, but limited by its adverse effects. Redox imbalance and reactive oxygen species generation plays major role in DOX-induced cardiotoxicity. Histamine, known to mediate various cardiovascular effects, but nevertheless the role of histamine or its receptors in DOX-induced DCM is remained obscure. Hence, this study is aimed to examine the effect of Famotidine (FAM), a H2 receptor antagonist on DOX-induced DCM in Wistar rats. Myocardial antioxidant status, stress and apoptosis markers, myocardial morphology and function were evaluated as the end points. Treatment with FAM has alleviated DOX doxorubicin-induced cardiotoxicity by reducing oxidative and nitrosative stress evident from lipid peroxidation and total nitrate-to-nitrite ratio, and enhanced the activity of super oxide dismutase. Cardiac stress markers like LDH and Na+-K+ATPase activities as well as CK-MB and Cardiac troponin levels were reduced by FAM treatment. It also normalised the myocardial function as assessed by 2D echocardiography and myocardial index. Treatment imparted anti-apoptotic effect as evident from decrease in myocardial caspase 3 and 9 activity and cleaved PARP expression. Effect of FAM is found to be comparable to the standard ACE inhibitor Captopril (CAP). The results from this study collectively suggest H2 receptor antagonism as a novel therapeutic strategy to impart biochemical, structural and functional improvement indicating its cardio-protective activity.







Similar content being viewed by others
References
Shakir DK, Rasul KI (2009) Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res 1:8–12. doi:10.4021/jocmr2009.02.1225
Biancaniello T, Meyer RA, Wong KY et al (1980) Doxorubicin cardiotoxicity in children. J Pediatr 97:45–50. doi:10.1016/S0022-3476(80)80128-4
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339:900–905. doi:10.1056/NEJM199809243391307
Octavia Y, Tocchetti CG, Gabrielson KL et al (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52:1213–1225. doi:10.1016/j.yjmcc.2012.03.006
Richard C, Ghibu S, Delemasure-Chalumeau S et al (2011) Oxidative stress and myocardial gene alterations associated with doxorubicin-induced cardiotoxicity in rats persist for 2 months after treatment cessation. J Pharmacol Exp Ther 339:807–814. doi:10.1124/jpet.111.185892
Gilleron M, Marechal X, Montaigne D et al (2009) NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis. Biochem Biophys Res Commun 388:727–731. doi:10.1016/j.bbrc.2009.08.085
Zhao Y, McLaughlin D, Robinson E et al (2010) Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res 70:9287–9297. doi:10.1158/0008-5472.CAN-10-2664
Ma J, Wang Y, Zheng D et al (2013) Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res 97:77–87. doi:10.1093/cvr/cvs309
van Acker SA, Kramer K, Grimbergen JA et al (1995) Monohydroxyethylrutoside as protector against chronic doxorubicin-induced cardiotoxicity. Br J Pharmacol 115:1260–1264
Decorti G, Klugmann FB, Candussio L, Baldini L (1989) Interaction of adriamycin with rat and mouse mast cells: histamine release and cellular uptake. Agents Actions 27:49–51
Dvorak AM (1986) Mast-cell degranulation in human hearts. N Engl J Med 315:969–970
Hara M, Ono K, Hwang M-W et al (2002) Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med 195:375–381
Del Valle J, Gantz I (1997) Novel insights into histamine H2 receptor biology. Am J Physiol 273:G987–G996
Potnuri AG, Allakonda L, Appavoo A et al (2016) Targeting histamine-2 receptor for prevention of cardiac remodelling in chronic pressure overload. Int J Cardiol 202:831–833. doi:10.1016/j.ijcard.2015.10.040
Luo T, Chen B, Zhao Z et al (2013) Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Res Cardiol 108:342. doi:10.1007/s00395-013-0342-4
Johnson CL, Weinstein H, Green JP (1979) Studies on histamine H2 receptors coupled to cardiac adenylate cyclase. Blockade by H2 and H1 receptor antagonists. Mol Pharmacol 16:417–428
Ahmadi A, Ebrahimzadeh MA, Ahmad-Ashrafi S et al (2011) Hepatoprotective, antinociceptive and antioxidant activities of cimetidine, ranitidine and famotidine as histamine H2 receptor antagonists. Fundam Clin Pharmacol 25:72–79. doi:10.1111/j.1472-8206.2009.00810.x
Pradeepkumar Singh L, Kundu P, Ganguly K et al (2007) Novel role of famotidine in downregulation of matrix metalloproteinase-9 during protection of ethanol-induced acute gastric ulcer. Free Radic Biol Med 43:289–299. doi:10.1016/j.freeradbiomed.2007.04.027
Khilnani G, Khilnani AK (2011) Inverse agonism and its therapeutic significance. Indian J Pharmacol 43:492–501. doi:10.4103/0253-7613.84947
Hayward R, Hydock DS (2007) Doxorubicin cardiotoxicity in the rat: an in vivo characterization. J Am Assoc Lab Anim Sci 46:20–32
Hou X-W, Jiang Y, Wang L-F et al (2009) Protective role of granulocyte colony-stimulating factor against adriamycin induced cardiac, renal and hepatic toxicities. Toxicol Lett 187:40–44. doi:10.1016/j.toxlet.2009.01.025
Mansour MA, El-Kashef HA, Al-Shabanah OA (1999) Effect of captopril on doxorubicin-induced nephrotoxicity in normal rats. Pharmacol Res Off J Ital Pharmacol Soc 39:233–237. doi:10.1006/phrs.1998.0432
Al-Shabanah O, Mansour M, El-Kashef H, Al-Bekairi A (1998) Captopril ameliorates myocardial and hematological toxicities induced by adriamycin. IUBMB Life 45:419–427. doi:10.1080/15216549800202802
Escudero EM, de Hurtado MCC, Pérez NG, Tufare AL (2004) Echocardiographic assessment of left ventricular midwall mechanics in spontaneously hypertensive rats. Eur Heart J Cardiovasc Imaging 5:169–175. doi:10.1016/j.euje.2003.11.004
Annapurna A, Challa SR, Prakash GJ, Viswanath RK (2008) Therapeutic potential of sulindac against ischemia-reperfusion-induced myocardial infarction in diabetic and nondiabetic rats. Exp Clin Cardiol 13:66–70
Potnuri AG, Kondru SK, Samudrala PK, Allakonda L (2017) Prevention of adriamycin induced cardiotoxicity in rats: a comparative study with subacute angiotensin-converting enzyme inhibitor and nonselective beta blocker therapy. IJC Metab Endocr 14:59–64. doi:10.1016/j.ijcme.2017.01.001
Fraga CG, Leibovitz BE, Tappel AL (1988) Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155–161. doi:10.1016/0891-5849(88)90023-8
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide Biol Chem Off J Nitric Oxide Soc 5:62–71. doi:10.1006/niox.2000.0319
Håkanson R, Rönnberg A (1974) Improved fluorometric assay of histamine: condensation with O-phthalaldehyde at −20 °C. Anal Biochem 60:560–567. doi:10.1016/0003-2697(74)90267-X
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Kaushal V, Herzog C, Haun RS, Kaushal GP (2014) Caspase protocols in mice. Methods Mol Biol Clifton NJ 1133:141–154. doi:10.1007/978-1-4939-0357-3_9
Sawamura I, Hazama F, Kinoshita M (1990) Histological and histometrical study of myocardial fibrosis in spontaneously hypertensive rats of the stroke-prone strain. Jpn Circ J 54:1274–1282
Wang XQ, Xiao AY, Sheline C et al (2003) Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. J Cell Sci 116:2099–2110. doi:10.1242/jcs.00420
Zhou S, Palmeira CM, Wallace KB (2001) Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett 121:151–157
Clements IP, Davis BJ, Wiseman GA (2002) Systolic and diastolic cardiac dysfunction early after the initiation of doxorubicin therapy: significance of gender and concurrent mediastinal radiation. Nucl Med Commun 23:521–527
Seif AE, Walker DM, Li Y et al (2015) Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatr Blood Cancer 62:704–709. doi:10.1002/pbc.25043
Biscotte SM, Levick SP, Bertling ML et al (2007) Angiotensin II mediated activation of cardiac mast cells. FASEB J 21:A1253–A1253
Reid AC, Brazin JA, Morrey C et al (2011) Targeting cardiac mast cells: pharmacological modulation of the local renin-angiotensin system. Curr Pharm Des 17:3744–3752
Sia YT, Lapointe N, Parker TG et al (2002) Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation 105:2549–2555
Berthiaume JM, Oliveira PJ, Fariss MW, Wallace KB (2005) Dietary vitamin E decreases doxorubicin-induced oxidative stress without preventing mitochondrial dysfunction. Cardiovasc Toxicol 5:257–267
Chakraborty H, Sen P, Sur A et al (2003) Age-related oxidative inactivation of Na+, K+-ATPase in rat brain crude synaptosomes. Exp Gerontol 38:705–710
Solomonson LP, Halabrin PR (1981) Cardiac sodium, potassium-adenosine triphosphatase as a possible site of adriamycin-induced cardiotoxicity. Cancer Res 41:570–572
Wu AHB (2006) Cardiac troponin friend of the cardiac physician, foe to the cardiac patient? Circulation 114:1673–1675. doi:10.1161/CIRCULATIONAHA.106.652123
Herman EH, Zhang J, Lipshultz SE et al (1999) Correlation between serum levels of cardiac troponin-T and the severity of the chronic cardiomyopathy induced by doxorubicin. J Clin Oncol Off J Am Soc Clin Oncol 17:2237–2243
Mair J, Apple F (1997) Progress in myocardial damage detection: new biochemical markers for clinicians. Crit Rev Clin Lab Sci 34:1–66. doi:10.3109/10408369709038215
Sato Y, Kita T, Takatsu Y, Kimura T (2004) Biochemical markers of myocyte injury in heart failure. Heart 90:1110–1113. doi:10.1136/hrt.2003.023895
Smith SH, Kramer MF, Reis I et al (1990) Regional changes in creatine kinase and myocyte size in hypertensive and nonhypertensive cardiac hypertrophy. Circ Res 67:1334–1344. doi:10.1161/01.RES.67.6.1334
Forman DE, Cittadini A, Azhar G et al (1997) Cardiac morphology and function in senescent rats: gender-related differences. J Am Coll Cardiol 30:1872–1877. doi:10.1016/S0735-1097(97)00411-7
Hutter JJ, Sahn DJ, Woolfenden JM, Carnahan Y (1981) Evaluation of the cardiac effects of doxorubicin by serial echocardiography. Am J Dis Child 135:653–657
Al-Biltagi M, Abd Rab Elrasoul Tolba O, El-Shanshory MR et al (2012) Strain echocardiography in early detection of doxorubicin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. Int Sch Res Not 2012:e870549. doi:10.5402/2012/870549
Zhao W, Zhao T, Chen Y et al (2008) Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem 317:43–50. doi:10.1007/s11010-008-9803-8
Abraham WT, Hayes DL (2003) Cardiac resynchronization therapy for heart failure. Circulation 108:2596–2603. doi:10.1161/01.CIR.0000096580.26969.9A
Ramani GV, Uber PA, Mehra MR (2010) Chronic heart failure: contemporary diagnosis and management. Mayo Clin Proc 85:180–195. doi:10.4065/mcp.2009.0494
Narula J, Haider N, Virmani R et al (1996) Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335:1182–1189. doi:10.1056/NEJM199610173351603
Hong BK, Kwon HM, Byun KH et al (2000) Apoptosis in dilated cardiomyopathy. Korean J Intern Med 15:56–64
Sanchez-Quintana D, Climent V, Garcia-Martinez V et al (1994) Extracellular matrix arrangement in the papillary muscles of the adult rat heart. Alterations after doxorubicin administration and experimental hypertension. Basic Res Cardiol 89:279–292
Xie M, Zhang W, Cheng TO et al (2013) Left ventricular torsion abnormalities in patients after the arterial switch operation for transposition of the great arteries with intact ventricular septum. Int J Cardiol 168:4631–4637. doi:10.1016/j.ijcard.2013.07.194
Acknowledgements
The authors would like to acknowledge the management of Sri Vishnu College of Pharmacy and Department of Cardiology, Andhra Medical College, Visakhapatnam for their support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors also disclose no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Kondru, S.K., Potnuri, A.G., Allakonda, L. et al. Histamine 2 receptor antagonism elicits protection against doxorubicin-induced cardiotoxicity in rodent model. Mol Cell Biochem 441, 77–88 (2018). https://doi.org/10.1007/s11010-017-3175-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3175-x