Abstract
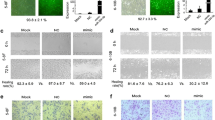
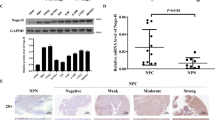
Nasopharyngeal carcinoma (NPC) is a unique subtype of head and neck cancer, with tendency to spread to regional lymph nodes and distant organs at early stage. Vimentin, a major cytoskeletal protein constituent of the intermediate filament, plays a critical role in the epithelial–mesenchymal transition. Overexpression of vimentin is considered to be a critical prerequisite for metastasis in numerous human cancers. Therefore, targeting vimentin for cancer therapy has gained a lot of interest. In the present study, we detected vimentin expression in NPC tissues and found that overexpression of vimentin is associated with poor prognosis of NPC patients. Silencing of vimentin in NPC CNE2 cells by RNAi suppresses cells migration and invasion in vitro. However, blocking vimentin did not affect cell proliferation of CNE2 cells. In addition, the in vivo metastatic potential of CNE2 cells transfected with Vimentin shRNA was suppressed in a nude mouse model of pulmonary metastasis. Silencing of Vimentin in CNE2 cells leads to a decrease of microvessel density and VEGF, CD31, MMP2, and MMP9 expressions in pulmonary metastatic tumors. Importantly, we found that it is easier for the tumor cells from the high vimentin-expressing pulmonary metastatic tumors to reinvade the microvessel and to form stable tumor plaques attached to the endothelial cells, which resemble the resource of circulating tumor cells and are very hard to eliminate. However, depletion of vimentin inhibits the formation of vascular tumor plaques. Our findings suggest that RNAi-based vimentin silencing may be a potential and promising anti-metastatic therapeutic strategy for NPC.






Similar content being viewed by others
References
Yan G, Lestari R, Long B, Fan Q, Wang Z, Guo X, Yu J, Hu J, Yang X, Chen C, Liu L, Li X, Purnomoadi A, Achmadi J, Yan X (2016) Comparative Proteomics Analysis Reveals l-Arginine Activates Ethanol Degradation Pathways in HepG2 Cells. Sci Rep 6:23340. doi:10.1038/srep23340
Sham JS, Choy D, Wei WI (1990) Nasopharyngeal carcinoma: orderly neck node spread. Int J Radiat Oncol Biol Phys 19(4):929–933
Pan TL, Wang PW, Huang CC, Yeh CT, Hu TH, Yu JS (2012) Network analysis and proteomic identification of vimentin as a key regulator associated with invasion and metastasis in human hepatocellular carcinoma cells. J Proteomics 75(15):4676–4692. doi:10.1016/j.jprot.2012.02.017
Helfand BT, Mendez MG, Murthy SN, Shumaker DK, Grin B, Mahammad S, Aebi U, Wedig T, Wu YI, Hahn KM, Inagaki M, Herrmann H, Goldman RD (2011) Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell 22(8):1274–1289. doi:10.1091/mbc.E10-08-0699
Satelli A, Li S (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci 68(18):3033–3046. doi:10.1007/s00018-011-0735-1
Mendez MG, Kojima S, Goldman RD (2010) Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 24(6):1838–1851. doi:10.1096/fj.09-151639
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146(5):1029–1039
Sanchez-Tillo E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A (2012) EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci 69(20):3429–3456. doi:10.1007/s00018-012-1122-2
Xiang B, Wang W, Li W, Li X, Li G (2012) Differential expression of oxidored nitro domain containing protein 1 (NOR1), in mouse tissues and in normal and cancerous human tissues. Gene 493(1):18–26. doi:10.1016/j.gene.2011.11.039
Xiang B, Yi M, Li W, Wang W, Zheng P, Li X, Li G (2014) Expression of oxidored nitro domain-containing protein 1(NOR1) impairs nasopharyngeal carcinoma cells adaptation to hypoxia and inhibits PDK1 expression. Mol Cell Biochem 393(1–2):293–300. doi:10.1007/s11010-014-2072-9
Wang W, Yi M, Chen S, Li J, Li G, Yang J, Zheng P, Zhang H, Xiong W, McCarthy JB, Li G, Li X, Xiang B (2016) Significance of the NOR1-FOXA1/HDAC2-Slug regulatory network in epithelial-mesenchymal transition of tumor cells. Oncotarget 7(13):16745–16759. doi:10.18632/oncotarget.7778
Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH (2007) Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res 67(8):3716–3724. doi:10.1158/0008-5472.CAN-06-4343
Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, Yao KT (2013) MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res 73(3):1219–1231. doi:10.1158/0008-5472.CAN-12-1408
Wang W, Li X, Zhang W, Li W, Yi M, Yang J, Zeng Z, Colvin Wanshura LE, McCarthy JB, Fan S, Zheng P, Chen S, Xiang B, Li G (2014) Oxidored-nitro domain containing protein 1 (NOR1) expression suppresses slug/vimentin but not snail in nasopharyngeal carcinoma: inhibition of EMT in vitro and in vivo in mice. Cancer Lett 348(1–2):109–118. doi:10.1016/j.canlet.2014.03.005
Yi M, Yang J, Li W, Li X, Xiong W, McCarthy JB, Li G, Xiang B (2017) The NOR1/OSCP1 proteins in cancer: from epigenetic silencing to functional characterization of a novel tumor suppressor. J Cancer 8(4):626–635. doi:10.7150/jca.17579
Dauphin M, Barbe C, Lemaire S, Nawrocki-Raby B, Lagonotte E, Delepine G, Birembaut P, Gilles C, Polette M (2013) Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer 81(1):117–122. doi:10.1016/j.lungcan.2013.03.011
Cheng Y, Zhou Y, Jiang W, Yang X, Zhu J, Feng D, Wei Y, Li M, Yao F, Hu W, Xiao W, Ling B (2012) Significance of E-cadherin, beta-catenin, and vimentin expression as postoperative prognosis indicators in cervical squamous cell carcinoma. Hum Pathol 43(8):1213–1220. doi:10.1016/j.humpath.2011.08.025
Zelenko Z, Gallagher EJ, Tobin-Hess A, Belardi V, Rostoker R, Blank J, Dina Y, LeRoith D (2017) Silencing vimentin expression decreases pulmonary metastases in a pre-diabetic mouse model of mammary tumor progression. Oncogene 36(10):1394–1403. doi:10.1038/onc.2016.305
Li XH, Chang H, Xu BQ, Tao YL, Gao J, Chen C, Qu C, Zhou S, Liu SR, Wang XH, Zhang WW, Yang X, Zhou SL, Xia YF (2017) An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med 6(1):310–319. doi:10.1002/cam4.947
Chang ET, Adami HO (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15(10):1765–1777. doi:10.1158/1055-9965.EPI-06-0353
Larsen JE, Nathan V, Osborne JK, Farrow RK, Deb D, Sullivan JP, Dospoy PD, Augustyn A, Hight SK, Sato M, Girard L, Behrens C, Wistuba II, Gazdar AF, Hayward NK, Minna JD (2016) ZEB1 drives epithelial-to-mesenchymal transition in lung cancer. J Clin Invest 126(9):3219–3235. doi:10.1172/JCI76725
Dmello C, Sawant S, Alam H, Gangadaran P, Mogre S, Tiwari R, D’Souza Z, Narkar M, Thorat R, Patil K, Chaukar D, Kane S, Vaidya M (2017) Vimentin regulates differentiation switch via modulation of keratin 14 levels and their expression together correlates with poor prognosis in oral cancer patients. PLoS ONE 12(2):e0172559. doi:10.1371/journal.pone.0172559
Jiu Y, Peranen J, Schaible N, Cheng F, Eriksson JE, Krishnan R, Lappalainen P (2017) Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J Cell Sci 130(5):892–902. doi:10.1242/jcs.196881
Woodham EF, Paul NR, Tyrrell B, Spence HJ, Swaminathan K, Scribner MR, Giampazolias E, Hedley A, Clark W, Kage F, Marston DJ, Hahn KM, Tait SW, Larue L, Brakebusch CH, Insall RH, Machesky LM (2017) Coordination by Cdc42 of Actin, Contractility, and Adhesion for Melanoblast Movement in Mouse Skin. Curr Biol 27(5):624–637. doi:10.1016/j.cub.2017.01.033
Riechelmann R, Grothey A (2017) Antiangiogenic therapy for refractory colorectal cancer: current options and future strategies. Ther Adv Med Oncol 9(2):106–126. doi:10.1177/1758834016676703
Ribatti D (2017) Epithelial-mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp Cell Res 353(1):1–5. doi:10.1016/j.yexcr.2017.02.041
Corominas D, Vollmer I, Paredes P (2017) Pulmonary Tumor Embolism due to Cardiac Metastasis of Lung Cancer. Arch Bronconeumol. doi:10.1016/j.arbres.2017.01.008
Acknowledgements
This study was supported in part by the Grants from The National Natural Science Foundation of China (81372304, 81572667, 81102065); the National “111” Project (Project #111-2-12); the Natural Science Foundation of Hunan Province, China (2015JJ2178); the Natural Science Foundation of Shandong Province, China (ZR2016HQ28); the Science and Technology Development Project of Jining ([2016] No. 56-28), and the Doctoral Fund of Affiliated Hospital of Jining Medical University (2016-BS-002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wang, W., Yi, M., Zhang, R. et al. Vimentin is a crucial target for anti-metastasis therapy of nasopharyngeal carcinoma. Mol Cell Biochem 438, 47–57 (2018). https://doi.org/10.1007/s11010-017-3112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3112-z