Abstract
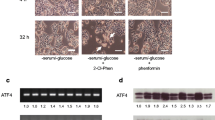
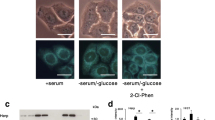
Recently, we developed a variety of phenformin derivatives as selective antitumor agents. Based on previous findings, this study evaluated a promising compound, 2-(2-chlorophenyl)ethylbiguanide (2-Cl-Phen), on the basis of stress responses in the human colon cancer cell line HT-29 under a serum- and glucose-deprived condition. 2-Cl-Phen triggered morphological changes such as shrinkage and plasma membrane disintegration, as well as a decrease in mitochondrial activity and an increase in LDH leakage. To understand intracellular issues relating to 2-Cl-Phen, this study focused on the expression levels of ER stress-inducible genes and several oncogenic genes. Serum and glucose deprivation significantly induced a variety of ER stress-inducible genes, but a 12-h treatment of 2-Cl-Phen down-regulated expression of several ER stress-related genes, with the exception of GADD153. Interestingly, the expression levels of ATF6α, GRP78, MANF, and CRELD2 mRNA were almost completely decreased by 2-Cl-Phen. This study also observed that a 24-h treatment of 2-Cl-Phen attenuated the expression levels of GRP78, GADD153, and c-Myc protein. The decrease in c-Myc protein occurred before the fluctuation of GRP78 protein, while the expression of c-Myc mRNA showed little change with cotreatment of serum and glucose deprivation with 2-Cl-Phen. To further understand the 2-Cl-Phen-induced down-regulation of ATF6-related genes, this study investigated the stability of ATF6α and GRP78 proteins using NanoLuc-tagged constructs. The expression levels of NanoLuc-tagged ATF6α and GRP78 were significantly down-regulated by 2-Cl-Phen in the presence or absence of the translation inhibitor cycloheximide. Taken together, our novel phenformin derivative 2-Cl-Phen has the unique characteristic of diminishing tumor adaptive responses, especially the expression of ATF6-related genes, as well as that of c-Myc protein, in a transcriptional and posttranscriptional manner under a serum- and glucose-deprived condition. Further characterization of cytotoxic mechanisms related to phenformin derivatives may give new insights into developing additional promising anticancer agents.







Similar content being viewed by others
Abbreviations
- ATF6:
-
Activating transcription factor 6
- CRELD2:
-
Cysteine-rich with EGF-like domains 2
- ER:
-
Endoplasmic reticulum
- GADD153:
-
Growth arrest and DNA damage-inducible protein 153
- GRP78:
-
78 kDa glucose-regulated protein
- GRP94:
-
94 kDa glucose-regulated protein
- G3PDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- MANF:
-
Mesencephalic astrocyte-derived neurotrophic factor
- XBP1:
-
X-box binding protein 1
References
Gething MJ, Sambrook J (1992) Protein folding in the cell. Nature 355:33–45
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030
Zhu C, Johansen FE, Prywes R (1997) Interaction of ATF6 and serum response factor. Mol Cell Biol 17:4957–4966
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459
Okada T, Yoshida H, Akazawa R, Negishi M, Mori K (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366:585–594
Rutkowski DT, Kaufman RJ (2003) All roads lead to ATF4. Dev Cell 4:442–444
Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, Fu Y, Luo B, Dubeau L, Hinton DR, Lee AS (2010) Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ 17:488–498
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Tomida A, Tsuruo T (1999) Drug resistance mediated by cellular stress response to the microenvironment of solid tumors. Anticancer Drug Des 14:169–177
Fu Y, Lee AS (2006) Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther 5:741–744
Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C (2005) ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 24:3470–3481
Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, Koumenis C (2012) ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest 122:4621–4634
Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS (2007) The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res 67:9809–9816
Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, Narita T, Masaki A, Ito A, Ding J, Kusumoto S, Ishida T, Komatsu H, Shiotsu Y, Ueda R, Iwawaki T, Imoto M, Iida S (2012) Identification of toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J 2:e79
Narise K, Okuda K, Enomoto Y, Hirayama T, Nagasawa H (2014) Optimization of biguanide derivatives as selective antitumor agents blocking adaptive stress responses in the tumor microenvironment. Drug Des Dev Ther 8:701–717
Oh-hashi K, Tanaka K, Koga H, Hirata Y, Kiuchi K (2012) Intracellular trafficking and secretion of mouse mesencephalic astrocyte-derived neurotrophic factor. Mol Cell Biochem 363:35–41
Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, Otto P, Zimmerman K, Vidugiris G, Machleidt T, Robers MB, Benink HA, Eggers CT, Slater MR, Meisenheimer PL, Klaubert DH, Fan F, Encell LP, Wood KV (2012) Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol 7:1848–1857
Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20:173–188
Oh-hashi K, Koga H, Ikeda S, Shimada K, Hirata Y, Kiuchi K (2009) CRELD2 is a novel endoplasmic reticulum stress-inducible gene. Biochem Biophys Res Commun 387:504–510
Sircar K, Huang H, Hu L, Cogdell D, Dhillon J, Tzelepi V, Efstathiou E, Koumakpayi IH, Saad F, Luo D, Bismar TA, Aparicio A, Troncoso P, Navone N, Zhang W (2012) Integrative molecular profiling reveals asparagine synthetase is a target in castration-resistant prostate cancer. Am J Pathol 180:895–903
van Riggelen J, Yetil A, Felsher DW (2010) MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 10:301–309
Babcock JT, Nguyen HB, He Y, Hendricks JW, Wek RC, Quilliam LA (2013) Mammalian target of rapamycin complex 1 (mTORC1) enhances bortezomib-induced death in tuberous sclerosis complex (TSC)-null cells by a c-MYC-dependent induction of the unfolded protein response. J Biol Chem 288:15687–15698
Amundson SA, Zhan Q, Penn LZ, Fornace AJ Jr (1998) Myc suppresses induction of the growth arrest genes gadd34, gadd45, and gadd153 by DNA-damaging agents. Oncogene 17:2149–2154
David CJ, Chen M, Assanah M, Canoll P, Manley JL (2010) HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463:364–368
Kern J, Untergasser G, Zenzmaier C, Sarg B, Gastl G, Gunsilius E, Steurer M (2009) GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood 114:3960–3967
Li Z, Wang Y, Wu H, Zhang L, Yang P, Li Z (2014) GRP78 enhances the glutamine metabolism to support cell survival from glucose deficiency by modulating the β-catenin signaling. Oncotarget 5:5369–5380
Harding HP, Zyryanova AF, Ron D (2012) Uncoupling proteostasis and development in vitro with a small molecule inhibitor of the pancreatic endoplasmic reticulum kinase. PERK J Biol Chem 287:44338–44344
Namba T, Ishihara T, Tanaka K, Hoshino T, Mizushima T (2007) Transcriptional activation of ATF6 by endoplasmic reticulum stressors. Biochem Biophys Res Commun 355:543–548
Ron D, Harding H (2007) eIF2α phosphorylation in cellular stress responses and disease. In: Sonenberg N, Hershey J, Mathews M (eds) Translational control., Cold Spring Harbor Monograph SeriesCold Spring Harbor Lab Press, Cold Spring Harbor, New York, pp 345–368
Nadanaka S, Okada T, Yoshida H, Mori K (2007) Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol 27:1027–1043
Higa A, Taouji S, Lhomond S, Jensen D, Fernandez-Zapico ME, Simpson JC, Pasquet JM, Schekman R, Chevet E (2014) Endoplasmic reticulum stress-activated transcription factor ATF6α requires the disulfide isomerase PDIA5 to modulate chemoresistance. Mol Cell Biol 34:1839–1849
Lee CY, Lee MG, Choi KC, Kang HM, Chang YS (2012) Clinical significance of GADD153 expression in stage I non-small cell lung cancer. Oncol Lett 4:408–412
Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, Ochoa AC, Cui Y, Del Valle L, Rodriguez PC (2014) The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 41:389–401
Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C (2010) The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J 29:2082–2096
Raykhel I, Alanen H, Salo K, Jurvansuu J, Nguyen VD, Latva-Ranta M, Ruddock L (2007) A molecular specificity code for the three mammalian KDEL receptors. J Cell Biol 179:1193–1204
Wasylishen AR, Chan-Seng-Yue M, Bros C, Dingar D, Tu WB, Kalkat M, Chan PK, Mullen PJ, Huang L, Meyer N, Raught B, Boutros PC, Penn LZ (2013) MYC phosphorylation at novel regulatory regions suppresses transforming activity. Cancer Res 73:6504–6515
Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R, Moll R, Elledge SJ, Eilers M (2007) The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol 9:765–774
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174
Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G (2010) Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11:390–401
Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Köhler A, Glossmann H, Schneider R, Sutherland C, Schweiger S (2010) Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA 107:21830–21835
Lee J, Chan SL, Lu C, Lane MA, Mattson MP (2002) Phenformin suppresses calcium responses to glutamate and protects hippocampal neurons against excitotoxicity. Exp Neurol 175:161–167
Acknowledgments
This work is partly supported by the Koshiyama Science and Technology Foundation (to Kensuke Okuda) and the OGAWA Science and Technology Foundation (to Kentaro Oh-hashi).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2016_2747_MOESM1_ESM.ppt
Supplementary material 1 (PPT 10203 kb) Supplementary Fig. 1 Effect of 2-Cl-Phen treatment on proliferation and damage in HT-29 cells cultured with the serum-containing or serum- and glucose-deprived medium. HT-29 cells in the serum-containing or serum- and glucose-deprived medium were treated with 2-Cl-Phen (50 μM) or vehicle for the indicated days. The arrows indicate the typical damaged cells
11010_2016_2747_MOESM2_ESM.pptx
Supplementary material 2 (PPTX 8425 kb) Supplementary Fig. 2 Expression of EGFP and NanoLuc-tagged ATF6α in COS7 cells A) The schematic structures of EGFP and NanoLuc-tagged ATF6α used in this study. Each construct has a Flag epitope (F) at the N-terminus. EGFP or NanoLuc (NL) gene was inserted between Flag tag and the N-terminus of full-length (FL) or nuclear localized (N) ATF6α. B) Forty-eight hours after transfection of the indicated construct into COS7 cells in a 6-well plate, fluorescent images were obtained by fluorescent microscopy (OLYMPUS). The scale bar is 100 μm. C) Forty-eight hours after transfection of the indicated construct into COS7 cells in a 6-well plate, the cells were lysed, and NanoLuc activity was measured using an aliquot of the lysate as described in the Materials and Methods section
11010_2016_2747_MOESM3_ESM.pptx
Supplementary material 3 (PPTX 8575 kb) Supplementary Fig. 3 Expression of EGFP and NanoLuc-tagged GRP78 in COS7 cells A) The schematic structures of EGFP and NanoLuc (NL)-tagged GRP78 (SP-EGFP-GRP78 and SP-NL-GRP78) used in this study. SP indicates a signal peptide sequence at the N-terminus. B) Forty-eight hours after transfection of the indicated construct into COS7 cells in a 6-well plate, fluorescent images were obtained by fluorescent microscopy. The scale bar is 100 μm. C) Forty-eight hours after transfection of the indicated construct into COS7 cells in a 6-well plate, the cells were lysed, and NanoLuc activity was measured using an aliquot of the lysate as described in the Materials and Methods section
Rights and permissions
About this article
Cite this article
Oh-hashi, K., Irie, N., Sakai, T. et al. Elucidation of a novel phenformin derivative on glucose-deprived stress responses in HT-29 cells. Mol Cell Biochem 419, 29–40 (2016). https://doi.org/10.1007/s11010-016-2747-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2747-5