Abstract
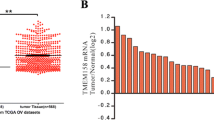
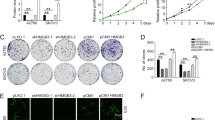
Ovarian cancer is one of the greatest causes of cancer death in women. The association of TMEM49 and ovarian cancer is poorly defined. Here, we reported that TMEM49 was significantly increased in ovarian tumor tissues compared to ovarian normal tissues. Furthermore, down-regulation of TMEM49 through RNA interference inhibited cell proliferation and arrested G1/S transition in two ovarian cancer cell lines, OVCAR3 and A2780. More importantly, TMEM49 silencing induced cell apoptosis. Additionally, down-regulation of TMEM49 in ovarian cancer notably repressed cell invasion and adhesion. Further gene set enrichment analysis suggested that apoptosis and metastasis up related signal pathways were associated with the TMEM49 expression. Western blot revealed that the expression of Caspase3, Bad, and Bax were increased, while expression of MMP2, KLF10, and CXCL12 were reduced by TMEM49 knockdown. Since expression of TMEM49 seems to be associated with the apoptosis and metastasis up signaling pathways of ovarian cancer, and suppression of its expression can inhibit cancer cell growth and metastasis, TMEM49 may be a potential therapeutic target in human ovarian cancer.







Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Banerjee S, Kaye SB (2013) New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res 19(5):961–968
Aunoble B, Sanches R, Didier E, Bignon Y (2000) Major oncogenes and tumor suppressor genes involved in epithelial ovarian cancer (review). Int J Oncol 16(3):567–643
Suzuki S, Moore DH, Ginzinger DG, Godfrey TE, Barclay J, Powell B, Pinkel D, Zaloudek C, Lu K, Mills G (2000) An approach to analysis of large-scale correlations between genome changes and clinical endpoints in ovarian cancer. Cancer Res 60(19):5382–5385
Pardo R, Lo Ré A, Archange C, Ropolo A, Papademetrio DL, Gonzalez CD, Alvarez EM, Iovanna JL, Vaccaro MI (2010) Gemcitabine induces the VMP1-mediated autophagy pathway to promote apoptotic death in human pancreatic cancer cells. Pancreatology 10(1):19–26
Molejon MI, Ropolo A, Vaccaro MI (2013) VMP1 is a new player in the regulation of the autophagy-specific phosphatidylinositol 3-kinase complex activation. Autophagy 9(6):933–935
Dusetti NJ, Jiang Y, Vaccaro MI, Tomasini R, Samir AA, Calvo EL, Ropolo A, Fiedler F, Mallo GV, Dagorn J-C (2002) Cloning and expression of the rat vacuole membrane protein 1 (VMP1), a new gene activated in pancreas with acute pancreatitis, which promotes vacuole formation. Biochem Biophys Res Commun 290(2):641–649
Guo L, Yang LY, Fan C, Chen GD, Wu F (2012) Novel roles of Vmp1: inhibition metastasis and proliferation of hepatocellular carcinoma. Cancer Sci 103(12):2110–2119
Grasso D, Ropolo A, Lo Re A, Boggio V, Molejon MI, Iovanna JL, Gonzalez CD, Urrutia R, Vaccaro MI (2011) Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem 286(10):8308–8324. doi:10.1074/jbc.M110.197301
Ropolo A, Grasso D, Pardo R, Sacchetti ML, Archange C, Re AL, Seux M, Nowak J, Gonzalez CD, Iovanna JL (2007) The pancreatitis-induced vacuole membrane protein 1 triggers autophagy in mammalian cells. J Biol Chem 282(51):37124–37133
Sauermann M, Sahin Ö, Sültmann H, Hahne F, Blaszkiewicz S, Majety M, Zatloukal K, Füzesi L, Poustka A, Wiemann S (2008) Reduced expression of vacuole membrane protein 1 affects the invasion capacity of tumor cells. Oncogene 27(9):1320–1326
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA 64(1):9–29
Grasso D, Sacchetti ML, Bruno L, Lo Re A, Iovanna JL, Gonzalez CD, Vaccaro MI (2009) Autophagy and VMP1 expression are early cellular events in experimental diabetes. Pancreatology 9(1–2):81–88. doi:10.1159/000178878
Qian Q, Zhou H, Chen Y, Shen C, He S, Zhao H, Wang L, Wan D, Gu W (2014) VMP1 related autophagy and apoptosis in colorectal cancer cells: vMP1 regulates cell death. Biochem Biophys Res Commun 443(3):1041–1047
Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP (2007) GSEA-P: a desktop application for gene set enrichment analysis. Bioinformatics 23(23):3251–3253
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6(2):99–104
Danial NN, Gramm CF, Scorrano L, Zhang C-Y, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB (2003) BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424(6951):952–956
Oltval ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 74(4):609–619
Gialeli C, Theocharis AD, Karamanos NK (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278(1):16–27
Subramaniam M, Hawse JR, Rajamannan NM, Ingle JN, Spelsberg TC (2010) Functional role of KLF10 in multiple disease processes. Biofactors 36(1):8–18
Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR (2002) Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res 62(20):5930–5938
Acknowledgments
Grant support was provided by Natural Science Foundation of Fujian Province, China (Grant No. 2013J01324).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Lili Zheng and Lingling Chen have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zheng, L., Chen, L., Zhang, X. et al. TMEM49-related apoptosis and metastasis in ovarian cancer and regulated cell death. Mol Cell Biochem 416, 1–9 (2016). https://doi.org/10.1007/s11010-016-2684-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2684-3