Abstract
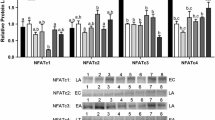
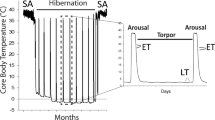
Over the course of the torpor-arousal cycle, hibernators must make behavioral, physiological, and molecular rearrangements in order to keep a very low metabolic rate and retain organ viability. 13-lined ground squirrels (Ictidomys tridecemlineatus) remain immobile during hibernation, and although the mechanisms of skeletal muscle survival are largely unknown, studies have shown minimal muscle loss in hibernating organisms. Additionally, the ground squirrel heart undergoes cold-stress, reversible cardiac hypertrophy, and ischemia–reperfusion without experiencing fatal impairment. This study examines the role of the Janus kinase–signal transducer and activator of transcription (JAK-STAT) signaling pathway in the regulation of cell stress in cardiac and skeletal muscles, comparing euthermic and hibernating ground squirrels. Immunoblots showed a fivefold decrease in JAK3 expression during torpor in skeletal muscle, along with increases in STAT3 and 5 phosphorylation and suppressors of cytokine signaling-1 (SOCS1) protein levels. Immunoblots also showed coordinated increases in STAT1, 3 and 5 phosphorylation and STAT1 inhibitor protein expression in cardiac muscle during torpor. PCR analysis revealed that the activation of these pro-survival signaling cascades did not result in coordinate changes in downstream genes such as anti-apoptotic B-cell lymphoma-2 (Bcl-2) family gene expression. Overall, these results indicate activation of the JAK-STAT pathway in both cardiac and skeletal muscles, suggesting a response to cellular stress during hibernation.











Similar content being viewed by others
References
Storey KB, Storey JM (2004) Mammalian hibernation: biochemical adaptation and gene expression. In: Storey KB (ed) Functional metabolism: regulation and adaptation. Wiley, Hoboken, pp 443–472
Storey KB (2010) Out cold: biochemical regulation of mammalian hibernation—a mini-review. Gerontology 56:220–230. doi:10.1159/000228829
Wang LCH, Lee TF (1996) Torpor and hibernation in mammals: metabolic, physiological, and biochemical adaptations. In: Handbook of physiology: environmental physiology, pp 507–532
Sheriff MJ, Fridinger RW, Tøien Ø et al (2013) Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool 86:515–527. doi:10.1086/673092
Cotton CJ, Harlow HJ (2010) Avoidance of skeletal muscle atrophy in spontaneous and facultative hibernators. Physiol Biochem Zool 83:551–560. doi:10.1086/650471
Tessier SN, Storey KB (2012) Myocyte enhancer factor-2 and cardiac muscle gene expression during hibernation in thirteen-lined ground squirrels. Gene 501:8–16. doi:10.1016/j.gene.2012.04.004
Storey KB, Storey JM (2000) Gene expression and protein adaptations in mammalian hibernation. In: Heldmaier G, Klingenspor M (eds) Life in the cold. Springer, New York, pp 303–313
Luu BE, Tessier SN, Duford DL, Storey KB (2015) The regulation of troponins I, C and ANP by GATA4 and Nk2–5 in heart of hibernating thirteen-lined ground squirrels, Ictidomys tridecemlineatus. PLoS ONE 10:e0117747. doi:10.1371/journal.pone.0117747
Wu CW, Storey KB (2014) FoxO3a-mediated activation of stress responsive genes during early torpor in a mammalian hibernator. Mol Cell Biochem. doi:10.1007/s11010-014-1969-7
Allan ME, Storey KB (2012) Expression of NF-kB and downstream antioxidant genes in skeletal muscle of hibernating ground squirrels, Spermophilus tridecemlineatus. Cell Biochem Funct 30:166–174. doi:10.1002/cbf.1832
Boengler K, Hilfiker-Kleiner D, Drexler H et al (2008) The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120:172–185. doi:10.1016/j.pharmthera.2008.08.002
Siveen KS, Sikka S, Surana R et al (2014) Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta 1845:136–154. doi:10.1016/j.bbcan.2013.12.005
Barry SP, Townsend PA, Latchman DS, Stephanou A (2007) Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med 13:82–89. doi:10.1016/j.molmed.2006.12.002
Trenerry MK, Gatta ADP, Cameron-Smith D (2011) JAK/STAT signaling and human in vitro myogenesis. BMC Physiol 11:6. doi:10.1186/1472-6793-11-6
Vendelbo MH, Jørgensen JO, Pedersen SB et al (2010) Exercise and fasting activate growth hormone-dependent myocellular signal transducer and activator of transcription-5b phosphorylation and insulin-like growth factor-I messenger ribonucleic acid expression in humans. J Clin Endocrinol Metab 95:64–68. doi:10.1210/jc.2010-0689
Cambi GE, Lucchese G, Djeokeng MMH et al (2012) Impaired JAK2-induced activation of STAT3 in failing human myocytes. Mol BioSyst 8:2351. doi:10.1039/c2mb25120e
Mascareno E, El-Shafei M, Maulik N et al (2001) JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation 104:325–329. doi:10.1161/01.CIR.104.3.325
Hattori R, Maulik N, Otani H et al (2001) Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol 33:1929–1936. doi:10.1006/jmcc.2001.1456
Nelson CJ, Otis JP, Carey HV (2009) A role for nuclear receptors in mammalian hibernation. J Physiol 587:1863–1870. doi:10.1113/jphysiol.2008.167692
McMullen DC, Hallenbeck JM (2010) Regulation of Akt during torpor in the hibernating ground squirrel, Ictidomys tridecemlineatus. J Comp Physiol B Biochem Syst Environ Physiol 180:927–934. doi:10.1007/s00360-010-0468-8
Pellissier F, Glogowskib CM, Heinemannb SF et al (2006) Lab assembly of a low-cost, robust SYBR green buffer system for quantitative real-time polymerase chain reaction. Anal Biochem 350:310–312. doi:10.1016/j.ab.2005.12.002
Schmittgen TD, Zakrajsek BA (2000) Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46:69–81. doi:10.1016/S0165-022X(00)00129-9
Dorritie KA, Redner RL, Johnson DE (2014) STAT transcription factors in normal and cancer stem cells. Adv Biol Regul. doi:10.1016/j.jbior.2014.05.004
Wickler SJ, Hoyt DF, van Breukelen F (1991) Disuse atrophy in the hibernating golden-mantled ground squirrel, Spermophilus lateralis. Am J Physiol 261:R1214–R1217
Ivakine EA, Cohn RD (2014) Maintaining skeletal muscle mass: lessons learned from hibernation. Exp Physiol 99:632–637. doi:10.1113/expphysiol.2013.074344
Grabek KR, Karimpour-Fard A, Epperson LE et al (2011) Multistate proteomics analysis reveals novel strategies used by a hibernator to precondition the heart and conserve ATP for winter heterothermy. Physiol Genomics 43:1263–1275. doi:10.1152/physiolgenomics.00125.2011
Biggar KK, Wu C-W, Tessier SN et al (2015) Modulation of gene expression in key survival pathways during daily torpor in the gray mouse lemur, Microcebus murinus. Genomics Proteomics Bioinform 13:111–118. doi:10.1016/j.gpb.2015.03.001
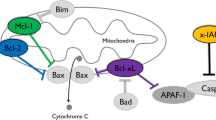
Rouble AN, Hefler J, Mamady H et al (2013) Anti-apoptotic signaling as a cytoprotective mechanism in mammalian hibernation. PeerJ 1:e29. doi:10.7717/peerj.29
Hindle AG, Grabek KR, Epperson LE et al (2014) Metabolic changes associated with the long winter fast dominate the liver proteome in 13-lined ground squirrels. Physiol Genomics 46:348–361. doi:10.1152/physiolgenomics.00190.2013
Stephanou A (2009) JAK-STAT pathway in disease. Landes Bioscience, Austin
Calò V, Migliavacca M, Bazan V et al (2003) STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol 197:157–168. doi:10.1002/jcp.10364
Wen Z, Darnell JE (1997) Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res 25:2062–2067. doi:10.1093/nar/25.11.2062
Shen Y, Schlessinger K, Zhu X et al (2004) Essential role of STAT3 in postnatal survival and growth revealed by mice lacking STAT3 serine 727 phosphorylation. Mol Cell Biol 24:407–419. doi:10.1128/MCB.24.1.407-419.2004
Gough DJ, Koetz L, Levy DE (2013) The MEK-ERK pathway is necessary for serine phosphorylation of mitochondrial STAT3 and ras-mediated transformation. PLoS ONE 8:1–9. doi:10.1371/journal.pone.0083395
Wen Z, Zhong Z, Darnell JE (1995) Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241–250. doi:10.1016/0092-8674(95)90311-9
Redell MS, Ruiz MJ, Alonzo T, Tweardy DJ (2010) Abstract 1791: Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation, impact on prognosis, and induction of apoptosis by a novel Stat3 inhibitor. Cancer Res 70:1791. doi:10.1158/1538-7445.AM10-1791
Wegrzyn J, Potla R, Chwae Y et al (2009) Function of mitochondrial STAT3 in cellular respiration. Science (80-) 323:793–797. doi:10.1126/science.1164551
Satoh JI, Tabunoki H (2013) A comprehensive profile of ChIP-Seq-based STAT1 target genes suggests the complexity of STAT1-mediated gene regulatory mechanisms. Gene Regul Syst Bio 2013:41–56. doi:10.4137/GRSB.S11433
Tamiya T, Kashiwagi I, Takahashi R et al (2011) Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol 31:980–985. doi:10.1161/ATVBAHA.110.207464
Imada K, Leonard WJ (2000) The Jak-STAT pathway. Mol Immunol 37:1–11
Yamaura G, Turoczi T, Yamamoto F et al (2003) STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H476–H482. doi:10.1152/ajpheart.00079.2003
Spierings D, McStay G, Saleh M et al (2005) Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science 310:66–67. doi:10.1126/science.1117105
Labi V, Erlacher M (2015) How cell death shapes cancer. Cell Death 6:e1675. doi:10.1038/cddis.2015.20
D’Amours D, Sallmann FR, Dixit VM, Poirier GG (2001) Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci 114:3771–3778
Wang K, Wang C, Xiao F et al (2008) JAK2/STAT2/STAT3 are required for myogenic differentiation. J Biol Chem 283:34029–34036. doi:10.1074/jbc.M803012200
Jang Y-N, Baik EJ (2013) JAK-STAT pathway and myogenic differentiation. Jak-Stat 2:e23282. doi:10.4161/jkst.23282
Sun L, Ma K, Wang H et al (2007) JAK1-STAT1-STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J Cell Biol 179:129–138. doi:10.1083/jcb.200703184
Consitt LA, Wideman L, Hickey MS, Morrison RF (2008) Phosphorylation of the JAK2-STAT5 pathway in response to acute aerobic exercise. Med Sci Sports Exerc 40:1031–1038. doi:10.1249/MSS.0b013e3181690760
Klover P, Chen W, Zhu B-M, Hennighausen L (2009) Skeletal muscle growth and fiber composition in mice are regulated through the transcription factors STAT5a/b: linking growth hormone to the androgen receptor. FASEB J 23:3140–3148. doi:10.1096/fj.08-128215
Kiu H, Nicholson SE (2012) Biology and significance of the JAK/STAT signalling pathways. Growth Factors 30:88–106. doi:10.3109/08977194.2012.660936
Al Zaid Siddiquee K, Turkson J (2008) STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 18:254–267. doi:10.1038/cr.2008.18
Subramaniam A, Shanmugam MK, Perumal E et al (2013) Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta: Rev Cancer 1835:46–60. doi:10.1016/j.bbcan.2012.10.002
Yu H, Jove R (2004) The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 4:97–105. doi:10.1038/nrc1275
Valentino L, Pierre J (2006) JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol 71:713–721. doi:10.1016/j.bcp.2005.12.017
Babon JJ, Kershaw NJ, Murphy JM et al (2013) Suppression of cyokine signalling by SOCS3: characterisation of the mode of inhibition and the basis of its specificity. Immunity 36:239–250. doi:10.1016/j.immuni.2011.12.015
Acknowledgments
We thank Dr. J. M. Hallenbeck at the NIH for providing ground squirrel tissues, Bryan E. Luu for advice and assistance with polymerase chain reaction and Luminex® assay preparation, and Jan Storey for editorial review of this manuscript. This work was supported by a Discovery Grant (#6793) from the Natural Sciences and Engineering Research Council of Canada and a grant from the Heart and Stroke Foundation of Canada (#G-14-0005874). KBS holds the Canada Research Chair in Molecular Physiology, SNT holds a NSERC Postdoctoral Fellowship, and SML held an NSERC Undergraduate Student Research Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Logan, S.M., Tessier, S.N., Tye, J. et al. Response of the JAK-STAT pathway to mammalian hibernation in 13-lined ground squirrel striated muscle. Mol Cell Biochem 414, 115–127 (2016). https://doi.org/10.1007/s11010-016-2665-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2665-6