Abstract
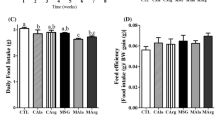
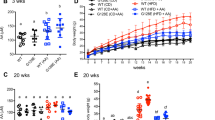
In this work, we aimed to investigate the effects of long-term supplementations with l-glutamine or l-alanyl-l-glutamine in the high-fat diet (HFD)-fed B6.129SF2/J mouse model over insulin sensitivity response and signaling, oxidative stress markers, metabolism and HSP70 expression. Mice were fed in a standard low-fat diet (STA) or a HFD for 20 weeks. In the 21th week, mice from the HFD group were allocated in five groups and supplemented for additional 8 weeks with different amino acids: HFD control group (HFD-Con), HFD + dipeptide l-alanyl-l-glutamine group (HFD-Dip), HFD + l-alanine group (HFD-Ala), HFD + l-glutamine group (HFD-Gln), or the HFD + l-alanine + l-glutamine (in their free forms) group (HFD-Ala + Gln). HFD induced higher body weight, fat pad, fasted glucose, and total cholesterol in comparison with STA group. Amino acid supplementations did not induce any modifications in these parameters. Although insulin tolerance tests indicated insulin resistance in all HFD groups, amino acid supplementations did not improve insulin sensitivity in the present model. There were also no significant differences in the immunocontents of insulin receptor, Akt, and Toll-like receptor-4. Notably, total 70 kDa heat shock protein (HSP72 + HSP73) contents in the liver was markedly increased in HFD-Con group as compared to STA group, which might suggest that insulin resistance is only in the beginning. Apparently, B6.129SF2/J mice are more resistant to the harmful effects of HFD through a mechanism that may include gut adaptation, reducing the absorption of nutrients, including amino acids, which may explain the lack of improvements in our intervention.





Similar content being viewed by others
References
Cho LW (2011) Metabolic syndrome. Singap Med J 52:779–785
Cheung WW, Mao P (2012) Recent advances in obesity: genetics and beyond. ISRN Endocrinol 2012:536905. doi:10.5402/2012/536905
Schwingshackl L, Hoffmann G (2014) Comparison of the long-term effects of high-fat v. low-fat diet consumption on cardiometabolic risk factors in subjects with abnormal glucose metabolism: a systematic review and meta-analysis. Br J Nutr 111:2047–2058. doi:10.1017/S0007114514000464
Newsholme P, Krause M (2014) Diet, obesity, and reactive oxygen species—implications for diabetes and aging. Syst Biol Free Radic Antioxid 01:3361–3374
Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, Becker W (2014) Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res. doi:10.3402/fnr.v58.25145
Rahati S, Shahraki M, Arjomand G, Shahraki T (2014) Food pattern, lifestyle and diabetes mellitus. Int J High Risk Behav Addict 3:e8725. doi:10.5812/ijhrba.8725
Jin C, Flavell RA (2013) Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol 132:287–294. doi:10.1016/j.jaci.2013.06.022
Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA (2015) Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes 4:598–612. doi:10.4239/wjd.v6.i4.598
Li H, Bao Y, Zhang X, Yu Y (2011) Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-κB pathway in rat aorta. Int J Cardiol 152:218–224. doi:10.1016/j.ijcard.2010.07.019
Newsholme P, Homem de Bittencourt PI Jr (2014) The fat cell senescence hypothesis: a mechanism responsible for abrogating the resolution of inflammation in chronic disease. Curr Opin Clin Nutr Metab Care 17:295–305. doi:10.1097/MCO.0000000000000077
Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, Homem de Bittencourt PI Jr (2015) The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat Inflamm. doi:10.1155/2015/249205
Krause M, Ludwig MS, Heck TG, Takahashi HK (2015) Heat shock proteins and heat therapy for type 2 diabetes: pros and cons. Curr Opin Clin Nutr Metab Care 18:374–380. doi:10.1097/MCO.0000000000000183
Krause M, Bock PM, Takahashi HK, Homem de Bittencourt PI, Jr Newsholme P (2015) The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci (Lond) 128:789–803. doi:10.1042/CS20140695
Henstridge DC, Whitham M, Febbraio MA (2014) Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol Metab 3:781–793. doi:10.1016/j.molmet.2014.08.003
Zachayus JL, Benatmane S, Plas C (1996) Role of Hsp70 synthesis in the fate of the insulin-receptor complex after heat shock in cultured fetal hepatocytes. J Cell Biochem 61:216–229
Liao Y, Hung MC (2010) Physiological regulation of Akt activity and stability. Am J Transl Res 2:19–42
Kurabe N, Mori M, Kurokawa J, Taniguchi K, Aoyama H, Atsuda K, Nishijima A, Odawara N, Harada S, Nakashima K, Arai S, Miyazaki T (2010) The death effector domain-containing DEDD forms a complex with Akt and Hsp90, and supports their stability. Biochem Biophys Res Commun 391:1708–1713. doi:10.1016/j.bbrc.2009.12.137
Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G (2012) Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones 17:293–302. doi:10.1007/s12192-011-0319-x
Henstridge DC, Forbes JM, Penfold SA, Formosa MF, Dougherty S, Gasser A, de Courten MP, Cooper ME, Kingwell BA, De Courten B (2010) The relationship between heat shock protein 72 expression in skeletal muscle and insulin sensitivity is dependent on adiposity. Metabolism 59:1556–1561. doi:10.1016/j.metabol.2010.01.027
Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD (2011) Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab 300:E894–E901. doi:10.1152/ajpendo.00699.2010
Petry ER, Cruzat VF, Heck TG, Leite JS, Homem de Bittencourt PI, Tirapegui J (2014) Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: involvement of heat shock protein pathways. Life Sci 94:130–136. doi:10.1016/j.lfs.2013.11.009
Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P (2005) Molecular mechanisms of glutamine action. J Cell Physiol 204:392–401. doi:10.1002/jcp.20339
Cruzat VF, Pantaleao LC, Donato J Jr, Homem de Bittencourt PI, Tirapegui J (2015) Oral supplementations with free and dipeptide forms of l-glutamine in endotoxemic mice: effects on muscle glutamine-glutathione axis and heat shock proteins. J Nutr Biochem 25:345–352. doi:10.1016/j.jnutbio.2013.11.009
Winzell MS, Ahrén B (2004) The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl 3):S215–S219
Opara EC, Petro A, Tevrizian A, Feinglos MN, Surwit RS (1996) l-Glutamine supplementation of a high fat diet reduces body weight and attenuates hyperglycemia and hyperinsulinemia in C57BL/6J mice. J Nutr 126:273–279
Gutierrez LL, Maslinkiewicz A, Curi R, Homem de Bittencourt PI Jr (2008) Atherosclerosis: a redox-sensitive lipid imbalance suppressible by cyclopentenone prostaglandins. Biochem Pharmacol 75:2245–2262. doi:10.1016/j.bcp.2008.03.002
Bezy O, Tran TT, Pihlajamäki J, Suzuki R, Emanuelli B, Winnay J, Mori MA, Haas J, Biddinger SB, Leitges M, Goldfine AB, Patti ME, King GL, Kahn CR (2011) PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J Clin Invest 121:2504–2517. doi:10.1172/JCI46045
Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, Homem de Bittencourt PI Jr (2015) Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity (Silver Spring) 23:120–129. doi:10.1002/oby.20919
Lund P (1986) l-Glutamine and l-glutamate: UV-method with glutaminase and glutamate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 3rd edn. Verlagsgesellschaft, Weinheim, pp 357–363
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Kolberg A, Rosa TG, Puhl MT, Scola G, da Rocha Janner D, Maslinkiewicz A, Lagranha DJ, Heck TG, Curi R, Homem de Bittencourt PI Jr (2006) Low expression of MRP1/GS-X pump ATPase in lymphocytes of Walker 256 tumour-bearing rats is associated with cyclopentenone prostaglandin accumulation and cancer immunodeficiency. Cell Biochem Funct 24:23–39. doi:10.1002/cbf.1290
Van Handel E (1965) Estimation of glycogen in small amounts of tissue. Anal Biochem 11:256–265
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Cruzat VF, Keane KN, Scheinpflug AL, Cordeiro R, Soares MJ, Newsholme P (2015) Alanyl-glutamine improves pancreatic beta-cell function following ex vivo inflammatory challenge. J Endocrinol 224:261–271. doi:10.1530/JOE-14-0677
Prada PO, Hirabara SM, de Souza CT, Schenka AA, Zecchin HG, Vassallo J, Velloso LA, Carneiro E, Carvalheira JB, Curi R, Saad MJ (2007) l-Glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle of rats with diet-induced obesity. Diabetologia 50:1949–1959. doi:10.1007/s00125-007-0723-z
Jacob PS, de Meneses Fujii TM, Yamada M, Borges MC, Pantaleão LC, Borelli P, Fock R, Rogero MM (2013) Isocaloric intake of a high-fat diet promotes insulin resistance and inflammation in Wistar rats. Cell Biochem Funct 31:244–253. doi:10.1002/cbf.2894
Wiśniewski JR, Friedrich A, Keller T, Mann M, Koepsell H (2015) The impact of high-fat diet on metabolism and immune defense in small intestine mucosa. J Proteome Res 14:353–365. doi:10.1021/pr500833v
Li G, Li J, Tan B, Wang J, Kong X, Guan G, Li F, Yin Y (2015) Characterization and regulation of the amino acid transporter SNAT2 in the small intestine of piglets. PLoS One 10:e0128207. doi:10.1371/journal.pone.0128207
Feng Z, Zhou X, Wu F, Yao K, Kong X, Li T, Blachier F, Yin Y (2014) Both dietary supplementation with monosodium l-glutamate and fat modify circulating and tissue amino acid pools in growing pigs, but with little interactive effect. PLoS One 9:e84533. doi:10.1371/journal.pone.0084533
Do TT, Hindlet P, Waligora-Dupriet AJ, Kapel N, Neveux N, Mignon V, Deloménie C, Farinotti R, Fève B, Buyse M (2014) Disturbed intestinal nitrogen homeostasis in a mouse model of high-fat diet-induced obesity and glucose intolerance. Am J Physiol Endocrinol Metab 306:E668–E680. doi:10.1152/ajpendo.00437.2013
De Castro Ghizoni CV, Gasparin FR, Júnior AS, Carreño FO, Constantin RP, Bracht A, Ishii Iwamoto EL, Constantin J (2013) Catabolism of amino acids in livers from cafeteria-fed rats. Mol Cell Biochem 373:265–277. doi:10.1007/s11010-012-1499-0
Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G (2009) Dietary l-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237. doi:10.3945/jn.108.096362
Benoit B, Plaisancié P, Awada M, Géloën A, Estienne M, Capel F, Malpuech-Brugère C, Debard C, Pesenti S, Morio B, Vidal H, Rieusset J, Michalski MC (2013) High-fat diet action on adiposity, inflammation, and insulin sensitivity depends on the control low-fat diet. Nutr Res 33:952–960. doi:10.1016/j.nutres.2013.07.017
Raso GM, Simeoli R, Iacono A, Santoro A, Amero P, Paciello O, Russo R, D’Agostino G, Di Costanzo M, Canani RB, Calignano A, Meli R (2014) Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J Nutr Biochem 25:81–90. doi:10.1016/j.jnutbio.2013.09.006
Sawada K, Ohtake T, Hasebe T, Abe M, Tanaka H, Ikuta K, Suzuki Y, Fujiya M, Hasebe C, Kohgo Y (2014) Augmented hepatic Toll-like receptors by fatty acids trigger the pro-inflammatory state of non-alcoholic fatty liver disease in mice. Hepatol Res 44:920–934. doi:10.1111/hepr.12199
Liu J, Zhuang ZJ, Bian DX, Ma XJ, Xun YH, Yang WJ, Luo Y, Liu YL, Jia L, Wang Y, Zhu ML, Ye DW, Zhou G, Lou GQ, Shi JP (2014) Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin Exp Pharmacol Physiol 41:482–488. doi:10.1111/1440-1681.12241
Krause M, Keane K, Rodrigues-Krause J, Crognale D, Egan B, De Vito G, Murphy C, Newsholme P (2014) Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro. Clin Sci (Lond) 126:739–752. doi:10.1042/CS20130678
Petry ER, Cruzat VF, Heck TG, Homem de Bittencourt PI, Tirapegui J (2015) l-Glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int J Sport Nutr Exerc Metab 25:188–197. doi:10.1123/ijsnem.2014-0131
Cruzat VF, Bittencourt A, Scomazzon SP, Leite JS, Homem de Bittencourt PI, Tirapegui J (2014) Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition 30:602–611. doi:10.1016/j.nut.2013.10.019
Cunningham GA, McClenaghan NH, Flatt PR, Newsholme P (2005) L-Alanine induces changes in metabolic and signal transduction gene expression in a clonal rat pancreatic beta-cell line and protects from pro-inflammatory cytokine-induced apoptosis. Clin Sci (Lond) 109:447–455
Menge BA, Schrader H, Ritter PR, Ellrichmann M, Uhl W, Schmidt WE, Meier JJ (2010) Selective amino acid deficiency in patients with impaired glucose tolerance and type 2 diabetes. Regul Pept 160:75–80. doi:10.1016/j.regpep.2009.08.001
Newsholme P, Abdulkader F, Rebelato E, Romanatto T, Pinheiro CH, Vitzel KF, Silva EP, Bazotte RB, Procopio J, Curi R, Gorjao R, Pithon-Curi TC (2011) Amino acids and diabetes: implications for endocrine, metabolic and immune function. Front Biosci (Landmark Ed) 16:315–339
Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN (2013) Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab 98:E1060–E1065. doi:10.1210/jc.2012-4132
RaS Marineli, Moura CS, Moraes É, Lenquiste SA, Lollo PC, Morato PN, Amaya-Farfan J, Maróstica MR (2015) Chia (Salvia hispanica L.) enhances HSP, PGC-1α expressions and improves glucose tolerance in diet-induced obese rats. Nutrition 31:740–748. doi:10.1016/j.nut.2014.11.009
Kavanagh K, Wylie AT, Chavanne TJ, Jorgensen MJ, Voruganti VS, Comuzzie AG, Kaplan JR, McCall CE, Kritchevsky SB (2012) Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J Gerontol Ser A 67:1014–1021. doi:10.1093/gerona/gls008
Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA (2014) Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63:1881–1894. doi:10.2337/db13-0967
Acknowledgments
We thank the Federal University of Rio Grande do Sul (UFRGS), Department of Physiology, for supporting this work. This work was partially supported by grants received from the Brazilian National Council for Scientific and Technological Development (CNPq, Grants from MCT/CNPq, MS/DECIT, CT-CIOTEC and CTSaúde, Grants #551097/2007-8, 402626/2012-5 and 402364/2012-0, to PIHBJ; 402398/2013-2 and 372373/2013-5 to MK).
Authors’ contributions
PMB, MK, HKT, and PIHBJ designed the study. PMB and HTS completed all the experiments described in this manuscript. GFH, MAB, MK, GN, LDZN, and MILR were involved in animal manipulation and studies on glycemic control and glutathione status. MK, HTS, GFH, GN, LDZN, and CMS performed molecular studies. PMB, MK, HTS, HKT, and PIHBJ analyzed the results. PMB, MK, and PIHBJ co-wrote the article. PIHBJ provided experimental advice and revised the final version of the manuscript. All the authors had final approval of the submitted and published versions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest and no competing interests such as consultancies, financial involvement, patent ownership, etc. in relation to the work described herein. CNPq (the funding organism) had no involvement in the propositions presented in this manuscript.
Ethical approval
All the procedures performed in studies involving the animals followed the ethical rules established by Arouca’s Act (Federal Law 11794/2008) and the Guide for Care and Use of Experimental Animals published by the National Institutes of Health (NIH publication no. 85-23, revised in 1996). The procedures were approved by the Federal University of Rio Grande do Sul Ethics Committee on Animal Experimentation (CEUA #21293/2011), according to the guidelines of the Brazilian National Council for the Control of Animal Experimentation (CONCEA).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bock, P.M., Krause, M., Schroeder, H.T. et al. Oral supplementations with l-glutamine or l-alanyl-l-glutamine do not change metabolic alterations induced by long-term high-fat diet in the B6.129F2/J mouse model of insulin resistance. Mol Cell Biochem 411, 351–362 (2016). https://doi.org/10.1007/s11010-015-2597-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2597-6