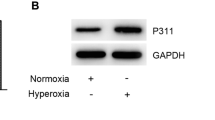
Abstract
Surfactant protein C (SP-C) deficiency is a risk factor for hyperoxia-induced bronchopulmonary dysplasia in newborn infants. However, the role of SP-C deficiency in the process is unclear. Here, using neonatal rat BPD model and MLE-12, mouse alveolar epithelial type II cell, we examined the changes of SP-C levels during hyperoxia. Immunohistochemistry, immunofluorescence, and ELISA analysis showed SP-C accumulation in alveolar epithelial type II cells. Electron microscopy further demonstrated the accumulation of lamellar bodies and the co-localization of lamellar bodies with autophagosomes in the cytoplasm of alveolar epithelial type II cells. The inhibition of autophagy with 3-Methyladenine and knockdown of Atg7 abolished hyperoxia-induced SP-C accumulation in the cytoplasm. Furthermore, inhibition of JNK signaling with SP600125 suppressed hyperoxia-induced Atg7 expression and SP-C accumulation. These findings suggest that hyperoxia triggers autophagy via JNK signaling-mediated Atg7 expression, which promotes the accumulation of SP-C within alveolar epithelial type II cells. Our data provide a potential approach for hyperoxic lung injury therapy by targeted pharmacological inhibition of autophagic pathway.





Similar content being viewed by others
References
Schober P, Schwarte LA (2012) From system to organ to cell: oxygenation and perfusion measurement in anesthesia and critical care. J Clin Monit Comput 26:255–265
Jobe AH, Bancalari E (2001) Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 163:1723–1729
Del Riccio V, Tuyl MV, Post M (2004) Apoptosis in lung development and neonatal lung injury. Pediatr Res 55:183–189
Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, Chen L, Yang XF, Ray A (2003) Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc Natl Acad Sci USA 100:6098–6103
Pagano A, Barazzone-Argiroffo C (2003) Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci 1010:405–416
Lyra PP, Diniz EM (2007) The importance of surfactant on the development of neonatal pulmonary diseases. Clinics (Sao Paulo) 62:181–190
Escande B, Kuhn P, Rivera S, Messer J (2004) Secondary surfactant deficiencies. Arch Pediatr 11:1351–1359
Chroneos ZC, Sever-Chroneos Z, Shepherd VL (2010) Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem 25:13–26
Tong Q, Zheng L, Dodd-o J, Langer J, Wang D, Li D (2006) Hypoxia-induced mitogenic factor modulates surfactant protein B and C expression in mouse lung. Am J Respir Cell Mol Biol 34:28–38
White CW, Greene KE, Allen CB, Shannon JM (2001) Elevated expression of surfactant proteins in newborn rats during adaptation to hyperoxia. Am J Respir Cell Mol Biol 25:51–59
Ter Horst SA, Fijlstra M, Sengupta S, Walther FJ, Wagenaar GT (2006) Spatial and temporal expression of surfactant proteins in hyperoxia-induced neonatal rat lung injury. BMC Pulm Med 6:8
Pérez-Gil J (2008) Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta 1778:1676–1695
You K, Xu X, Fu J, Xu S, Yue X, Yu Z, Xue X (2012) Hyperoxia disrupts pulmonary epithelial barrier in newborn rats via the deterioration of occludin and ZO-1. Respir Res 13:36
Lee JA, Gao FB (2009) Inhibition of autophagy induction delays neuronal cell loss caused by dysfunctional ESCRT-III in frontotemporal dementia. J Neurosci 29:8506–8511
Gururajan M, Chui R, Karuppannan AK, Ke J, Jennings CD, Bondada S (2005) c-Jun N-terminal kinase (JNK) is required for survival and proliferation of B-lymphoma cells. Blood 106:1382–1391
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441:523–540
McClung JM, Judge AR, Powers SK, Yan Z (2010) p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 298:C542–C549
Orgeig S, Hiemstra PS, Veldhuizen EJ, Casals C, Clark HW, Haczku A, Knudsen L, Possmayer F (2010) Recent advances in alveolar biology: evolution and function of alveolar proteins. Respir Physiol Neurobiol 73(Suppl):S43–54
Osborn DA, Hunt RW (2007) Postnatal thyroid hormones for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev 24:CD005946
Koslowski R, Kasper M, Schaal K, Knels L, Lange M, Bernhard W (2013) Surfactant metabolism and anti-oxidative capacity in hyperoxic neonatal rat lungs: effects of keratinocyte growth factor on gene expression in vivo. Histochem Cell Biol 139:461–472
Bein K, Di Giuseppe M, Mischler SE, Ortiz LA, Leikauf GD (2013) LPS-treated acrophage cytokines repress surfactant protein-B in lung epithelial cells. Am J Respir Cell Mol Biol 49:306–315
Sugahara K, Iyama K, Sano K, Kuroki Y, Akino T, Matsumoto M (1996) Overexpression of surfactant protein SP-A, SP-B and SP-C mRNA in rat lungs with lipopolysaccharide induced injury. Lab Invest 74:209–220
Dombrowsky H, Tschernig T, Vieten G, Rau GA, Ohler F, Acevedo C, Behrens C, Poets CF, von der Hardt H, Bernhard W (2006) Molecular and functional changes of pulmonary surfactant in response to hyperoxia. Pediatr Pulmonol 41:1025–1039
Fehrenbach H, Brasch F, Uhlig S, Weisser M, Stamme C, Wendel A, Richter J (1998) Early alterations in intracellular and alveolar surfactant of the rat lung in response to endotoxin. Am J Respir Crit Care Med 157:1630–1639
Domenici L, Pieri L, Gallè MB, Romagnoli P, Adembri C (2004) Evolution of endotoxin-induced lung injury in the rat beyond the acute phase. Pathobiology 71:59–69
Jin Y, Tanaka A, Choi AM, Ryter SW (2012) Autophagic proteins: new facets of the oxygen paradox. Autophagy 8:426–428
Ryter SW, Choi AM (2013) Regulation of autophagy in oxygen-dependent cellular stress. Curr Pharm Des 19:2747–2756
Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H (2008) Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab 8:325–332
Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS (2008) Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab 8:318–324
Ushio H, Ueno T, Kojima Y, Komatsu M, Tanaka S, Yamamoto A, Ichimura Y, Ezaki J, Nishida K, Komazawa-Sakon S, Niyonsaba F, Ishii T, Yanagawa T, Kominami E, Ogawa H, Okumura K, Nakano H (2011) Crucial role for autophagy in degranulation of mast cells. J. Allergy Clin. Immunol 127(1267–1276):e6
DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, Klumperman J, Tooze SA, Teitelbaum SL, Virgin HW (2011) Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell 21:966–974
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81170605).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Zhao, S., Yuan, LJ. et al. Autophagy regulates hyperoxia-induced intracellular accumulation of surfactant protein C in alveolar type II cells. Mol Cell Biochem 408, 181–189 (2015). https://doi.org/10.1007/s11010-015-2494-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2494-z