Abstract
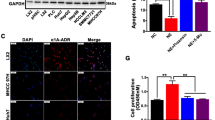
The emerging role of stress-related signaling in regulating cancer development and progression has been recognized. However, whether stress serves as a mechanism to promote gastric cancer metastasis is not clear. Here, we show that the β2-AR agonist, isoprenaline, upregulates expression levels of CD44 and CD44v8-10 in gastric cancer cells. CD44, a cancer stem cell-related marker, is expressed at high levels in gastric cancer tissues, which strongly correlates with the occurrence of epithelial–mesenchymal transition (EMT)-associated phenotypes both in vivo and in vitro. Combined with experimental observations in two human gastric cancer cell lines, we found that β2-AR signaling can initiate EMT. It led to an increased expression of mesenchymal markers, such as α-SMA, vimentin, and snail at mRNA and protein levels, and conversely a decrease in epithelial markers, E-cadherin and β-catenin. Isoprenaline stimulation of β2-AR receptors activates the downstream target STAT3, which functions as a positive regulator and mediated the phenotypic switch toward a mesenchymal cell type in gastric cancer cells. Our data provide a mechanistic understanding of the complex signaling cascades involving stress-related hormones and their effects on EMT. In light of our observations, pharmacological interventions targeting β2-AR-STAT3 signaling can potentially be used to ameliorate stress-associated influences on gastric cancer development and progression.









Similar content being viewed by others
References
Lozano R et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2095–2128
Price MA et al (2001) The role of psychosocial factors in the development of breast carcinoma: part II. Life event stressors, social support, defense style, and emotional control and their interactions. Cancer 91(4):686–697
Price MA et al (2001) The role of psychosocial factors in the development of breast carcinoma: part I. The cancer prone personality. Cancer 91(4):679–685
Reiche EM, Nunes SO, Morimoto HK (2004) Stress, depression, the immune system, and cancer. Lancet Oncol 5(10):617–625
Antoni MH et al (2006) The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6(3):240–248
Sood AK et al (2010) Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 120(5):1515–1523
Thaker PH et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12(8):939–944
Shi M et al (2011) The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat 125(2):351–362
Shi M et al (2013) Catecholamine-induced beta2-adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J Immunol 190(11):5600–5608
Shi M et al (2010) Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer 9:269
Liu H et al (2010) Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci USA 107(42):18115–18120
Liu R et al (2007) The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 356(3):217–226
Mani SA et al (2008) The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Yang J, Weinberg RA (2008) Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14(6):818–829
Rhim AD et al (2012) EMT and dissemination precede pancreatic tumor formation. Cell 148(1–2):349–361
Landen CJ et al (2007) Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res 67(21):10389–10396
Rokavec M et al (2014) IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest 124(4):1853–1867
Liu RY et al (2014) JAK/STAT3 signaling is required for TGF-beta-induced epithelial–mesenchymal transition in lung cancer cells. Int J Oncol 44(5):1643–1651
Gregory PA et al (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10(5):593–601
Shorning BY, Griffiths D, Clarke AR (2011) Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial–mesenchymal transition in the mouse bladder. PLoS ONE 6(1):e16209
Noseda M et al (2004) Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res 94(7):910–917
Sanchez-Tillo E et al (2012) EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci 69(20):3429–3456
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196
Iseri OD et al (2014) Beta-adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharm Biol 52(11):1374–1381
Acknowledgments
This work was supported by Natural Science Foundation of China, No. 30901564, No. 81101883, No. 81372067, No. 81121004, No. 81230041; National Basic Science and Development Programme (973 Programme) No. 2012CB518105; Beijing Novel Program, No. 2008B53, No. 2009A38; Science and technology support program of Hebei Province of China, No. 13277779D; and the Key Subjects in Universities and Colleges of Hebei Province of China (pathology and pathophysiology).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Yan-Jie Lu and Zhi-Jun Geng have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
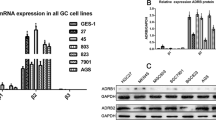
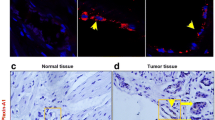
CD44 expressed negatively correlated with β-catenin in gastric cancer.Representative fluorescence images showing the double-labeling of CD44 withepithelial marker β-catenin in peri-cancerous (A), cancerous (B) and metastatic gastriccancer tissue samples (C). In normal gastric mucosa epithelial cells β-catenin washighly expressed (A) and downregulated in cancerous tissue (B) and lymphaticmetastasis (C). In cancerous tissue where CD44 was expressed at high levels, theexpression of β-catenin was downregulated and was inversely correlated with thedistribution patterns of CD44. In contrast, in cancerous tissues where CD44 showed lowexpression, β-catenin was expressed at high levels in the membrane of cancerous gastricepithelial cells. A similar result can be obtained in lymphatic metastasis that CD44 washighly expressed and β-catenin expression was nearly absent. A–C, Bar 25 μm. (TIFF 14184 kb)
Rights and permissions
About this article
Cite this article
Lu, YJ., Geng, ZJ., Sun, XY. et al. Isoprenaline induces epithelial–mesenchymal transition in gastric cancer cells. Mol Cell Biochem 408, 1–13 (2015). https://doi.org/10.1007/s11010-015-2477-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2477-0