Abstract
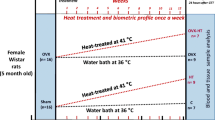
Hot flashes, which involve a tiny rise in core temperature, are the most common complaint of peri- and post-menopausal women, being tightly related to decrease in estrogen levels. On the other hand, estradiol (E2) induces the expression of HSP72, a member of the 70 kDa family of heat shock proteins (HSP70), which are cytoprotective, cardioprotective, and heat inducible. Since HSP70 expression is compromised in age-related inflammatory diseases, we argued whether the capacity of triggering a robust heat shock (HS) response would be still present after E2 withdrawal. Hence, we studied the effects of HS treatment (hot tub) in female Wistar rats subjected to bilateral ovariectomy (OVX) after a 7-day washout period. Twelve h after HS, the animals were killed and aortic arches were surgically excised for molecular analyses. The results were compared with oxidative stress markers in the plasma (superoxide dismutase, catalase, and lipoperoxidation) because HSP70 expression is also sensitive to redox regulation. Extracellular (plasma) to intracellular HSP70 ratio, an index of systemic inflammatory status, was also investigated. The results showed that HS response was preserved in OVX animals, as inferred from HSP70 expression (up to 40 % rise, p < 0.01) in the aortas, which was accompanied by no further alterations in oxidative stress, hematological parameters, and glycemic control either. This suggests that the lack of estrogen per se could not be solely ascribed as the unique source of low HSP70 expression as observed in long-term post-menopausal individuals. As a consequence, periodic evaluation of HSP70 status (iHSP70 vs. eHSP70) may be of clinical relevance because decreased HS response capacity is at the center of the onset of menopause-related dysfunctions.



Similar content being viewed by others
Abbreviations
- HS:
-
Heat shock
- HSP70:
-
The 70 kDa family of heat shock proteins
- E2:
-
Estradiol
References
Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D (2002) Hot flushes. Lancet 360:1851–1861
Dacks PA, Rance NE (2010) Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology 151:1187–1193. doi:10.1210/en.2009-1112
Hamilton KL, Gupta S, Knowlton AA (2004) Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NF kappa B signaling. J Mol Cell Cardiol 36:577–584
Hamilton KL, Mbai FN, Gupta S, Knowlton AA (2004) Estrogen, heat shock proteins, and NF-κB in human vascular endothelium. Arterioscler Thromb Vasc Biol 24:1628–1633
Stice JP, Knowlton AA (2008) Estrogen, NF-κB, and the heat shock response. Mol Med 14:517–527. doi:10.2119/2008-00026
Stice JP, Chen L, Kim SC, Jung JS, Tran AL, Liu TT, Knowlton AA (2011) 17β-Estradiol, aging, inflammation, and the stress response in the female heart. Endocrinology 152:1589–1598. doi:10.1210/en.2010-0627
Ritossa F (1962) A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 18:571–573
Heck TG, Scholer CM, de Bittencourt PI (2011) HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct 29:215–226. doi:10.1002/cbf.1739
Ludwig MS, Minguetti-Câmara VC, Heck TG, Scomazzon SP, Nunes PR, Bazotte RB, Homem de Bittencourt PI Jr (2014) Short-term but not long-term hypoglycaemia enhances plasma levels and hepatic expression of HSP72 in insulin-treated rats: an effect associated with increased IL-6 levels but not with IL-10 or TNFα. Mol Cell Biochem 397:97–107. doi:10.1007/s11010-014-2176-2
Singh IS, Hasday JD (2013) Fever, hyperthermia and the heat shock response. Int J Hyperthermia 29:423–435. doi:10.3109/02656736.2013.808766
de Thonel A, Le Mouël A, Mezger V (2012) Transcriptional regulation of small HSP-HSF1 and beyond. Int J Biochem Cell Biol 44:1593–1612. doi:10.1016/j.biocel.2012.06.012
Tang S, Buriro R, Liu Z, Zhang M, Ali I, Adam A, Hartung J, Bao E (2013) Localization and expression of Hsp27 and αB-crystallin in rat primary myocardial cells during heat stress in vitro. PLoS One 8:e69066. doi:10.1371/journal.pone.0069066
Anckar J, Sistonen L (2011) Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80:1089–1115. doi:10.1146/annurev-biochem-060809-095203
Newsholme P, Homem de Bittencourt PI Jr (2014) The fat cell senescence hypothesis: a mechanism responsible for abrogating the resolution of inflammation in chronic disease. Curr Opin Clin Nutr Metab Care 17:295–305. doi:10.1097/MCO.0000000000000077
Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA (2003) Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol 285:H687–H692
Milne KJ, Thorp DB, Melling CW, Noble EG (2006) Castration inhibits exercise-induced accumulation of Hsp70 in male rodent hearts. Am J Physiol Heart Circ Physiol 290:H1610–H1616
Papaconstantinou AD, Fisher BR, Umbreit TH, Goering PL, Lappas NT, Brown KM (2001) Effects of beta-estradiol and bisphenol A on heat shock protein levels and localization in the mouse uterus are antagonized by the antiestrogen ICI 182,780. Toxicol Sci 63:173–180
Bombardier E, Vigna C, Bloemberg D, Quadrilatero J, Tiidus PM, Tupling AR (2013) The role of estrogen receptor-α in estrogen-mediated regulation of basal and exercise-induced Hsp70 and Hsp27 expression in rat soleus. Can J Physiol Pharmacol 91:823–829
Goloubkova T, Ribeiro MF, Rodrigues LP, Cecconello AL, Spritzer PM (2000) Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch Toxicol 74:92–98
Knapp RT, Wong MJ, Kollmannsberger LK, Gassen NC, Kretzschmar A, Zschocke J, Hafner K, Young JC, Rein T (2014) Hsp70 cochaperones HspBP1 and BAG-1 M differentially regulate steroid hormone receptor function. PLoS One 9:e85415. doi:10.1371/journal.pone.0085415
Ahn SG, Thiele DJ (2003) Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev 17:516–528
Zou W (2011) Ovariectomy (oophorectomy). Protoc Exch. doi:10.1038/protex.2011.242
Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA (2008) HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 105:1739–1744. doi:10.1073/pnas.0705799105
Brown ET, Umino Y, Loi T, Solessio E, Barlow R (2005) Anesthesia can cause sustained hyperglycemia in C57/BL6 J mice. Vis Neurosci 22:615–618. doi:10.1017/S0952523805225105
Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA (2005) Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med (Maywood) 230:777–784
Chang Y, Chen TL, Sheu JR, Chen RM (2005) Suppressive effects of ketamine on macrophage functions. Toxicol Appl Pharmacol 204:27–35. doi:10.1016/j.taap.2004.08.011
Febbraio MA, Mesa JL, Chung J, Steensberg A, Keller C, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK (2004) Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperon 9:390–396
Bedell SE, Bush BT (1985) Erythrocyte sedimentation rate. From folklore to facts. Am J Med 78(6):1001–1009
Homem de Bittencourt PI Jr, Lagranha DJ, Maslinkiewicz A, Senna SM, Tavares AMV, Baldissera P, Janner DR, Peralta JS, Bock PM, Gutierrez LLP, Scola G, Heck TG, Krause MS, Cruz LA, Abdalla DSP, Lima T, Curi R (2007) LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis 193:245–258. doi:10.16/j.atherosclerosis.2006.08.049
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1978) Microssomal lipid peroxidation. Methods Enzymol 52:302–309
Marklund S, Marklung G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121
Kolberg A, Rosa TG, Puhl MT, Scola G, da Rocha Janner D, Maslinkiewicz A, Lagranha DJ, Heck TG, Curi R, de Bittencourt PI Jr (2006) Low expression of MRP1/GS-X pump ATPase in lymphocytes of Walker 256 tumour-bearing rats is associated with cyclopentenone prostaglandin accumulation and cancer immunodeficiency. Cell Biochem Funct 24:23–39. doi:10.1002/cbf.1290
Krause MS, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, Homem de Bittencourt PI Jr (2015) The chaperone balance hypothesis: the importance of the Extracellular to Intracellular HSP70 Ratio (eHSP70/iHSP70) to inflammation-driven type 2 Diabetes, the effect of exercise and the implications for clinical management. Mediat Inflamm 2015:249205. doi:10.1155/2015/249205
Krause MS, Bock PM, Takahashi HK, Homem de Bittencourt PI Jr, Newsholme P (2015) The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci (London) 128:789–803. doi:10.1042/CS20140695
Wassmann S, Bäumer AT, Strehlow K, van Eickels M, Grohé C, Ahlbory K, Rösen R, Böhm M, Nickenig G (2001) Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation 103:435–441
Zimon A, Erat A, Von Wald T, Bissell B, Koulova A, Choi CH, Bachvarov D, Reindollar RH, Usheva A (2006) Genes invoked in the ovarian transition to menopause. Nucleic Acids Res 34:3279–3287
Yoon SJ, Choi KH, Lee KA (2002) Nitric oxide-mediated inhibition of follicular apoptosis is associated with HSP70 induction and Bax suppression. Mol Reprod Dev 61:504–510
Kim AH, Khanna A, Aten RF, Olive DL, Behrman HR (1996) Cytokine induction of heat shock protein in human granulosa-luteal cells. Mol Hum Reprod 2:549–554
Ridker PM (2001) High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 103:1813–1818
Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ (2002) A self-fulfilling prophecy C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 106:913–919
Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L (2004) C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation 109:647–655 (Erratum in: Circulation. 2004 May 11;109(18):2254)
Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G (2012) Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperon 17:293–302. doi:10.1007/s12192-011-0319-x
Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, Homem de Bittencourt PI Jr (2015) Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity 23:120–129. doi:10.1002/oby.20919
Chichester L, Wylie AT, Craft S, Kavanagh K (2015) Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci 70:155–162. doi:10.1093/gerona/glu015
Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R (2000) Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 106:1229–1237
Krause MS, Nakajima ST (2015) Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am 42:163–179. doi:10.1016/j.ogc.2014.09.008
Rossato JS, Krause MS, Fernandes AJ, Fernandes JR, Seibt IL, Rech A, Homem de Bittencourt PI Jr (2014) Role of alpha- and beta-adrenoreceptors in rat monocyte/macrophage function at rest and acute exercise. J Physiol Biochem 70:363–374. doi:10.1007/s13105-013-0310-3
Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M (1985) Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J Appl Physiol 99:1789–1795
Acknowledgments
This work was partially supported by grants received from the Brazilian National Council for Scientific and Technological Development (CNPq) #563870/2010-9, 402626/2012-5, and 402364/2012-0 to PIHBJ. TGH was supported by grants from CNPq (382692/2011-0) and the State of Rio Grande Foundation for Research Support (FAPERGS, 002106-2551/13-5). AAM, MSL, TGH, and PIHBJ designed the study. AAM, MSL, and TGH completed all the experiments described in this manuscript. FGB performed HS treatments, glucose status monitoring experiments, and Western analyses. Oxidative stress studies were conducted by ABS. MNF was involved in hematology and ELISA analyses. All authors were involved in analyzing the results. PIHBJ provided experimental advice and wrote the paper. All the authors had final approval of the submitted and published versions.
Ethical approval
The procedures described herein were approved by the Federal University of Rio Grande do Sul Ethics Committee on Animal Experimentation (CEUA #19858), according to the guidelines of the Brazilian National Council for the Control of Animal Experimentation (CONCEA).
Conflict of interest
The authors declare no conflict of interest and no competing interests such as consultancies, financial involvement, and patent ownership in relation to the work described.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miragem, A.A., Ludwig, M.S., Heck, T.G. et al. Estrogen deprivation does not affect vascular heat shock response in female rats: a comparison with oxidative stress markers. Mol Cell Biochem 407, 239–249 (2015). https://doi.org/10.1007/s11010-015-2472-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2472-5