Abstract
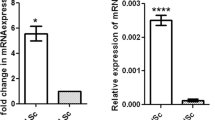
SRY-related box (Sox) transcription factors are conserved among vertebrate species. These proteins regulate multiple processes including sex determination and testis differentiation of the male embryo. Although members of the Sox family have been identified in pre- and postnatal Sertoli cells, they have never been characterized in adult Leydig cells. The objectives of this research were to identify expressions of Sox9, Sox5, and Sox13 in mice Leydig cell cultures and to establish their expression profiles in postnatal mice testes at different developmental stages. Methods used include Western blots and qPCR of stimulated MA-10 cell cultures and whole mice testes. Sox9, Sox5, and Sox13 proteins were detected in MA-10 cells as well as whole mouse testis. Although Sox9, Sox5, and Sox13 mRNA levels from whole mice testes tended to increase according to postnatal development, these results were not significant. Sox members were also detected in whole mice testis by Western Blot. However, Sox9, Sox5, and Sox13 protein expressions remained relatively constant during postnatal development from postnatal (P) day 60 to P365. Being newly characterized in the mouse testis, Sox13 was mainly localized by immunofluorescence within the nuclei of cells from seminiferous tubules, possibly spermatocytes and Sertoli cells. In addition, Sox9, Sox5, and Sox13 proteins were characterized in the nuclei of MA-10 Leydig cell cultures. Their expressions and transcriptional activities remained unaffected by activators of the cAMP/PKA pathway. Thus, Sox9, Sox5, and Sox13 transcription factors are expressed in postnatal testis and may regulate multiple functions such as steroidogenesis and spermatogenesis.






Similar content being viewed by others
References
Schepers GE, Teasdale RD, Koopman P (2002) Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell 3:167–170
Grosschedl R, Giese K, Pagel J (1994) HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet TIG 10:94–100
Gubbay J, Collignon J, Koopman P et al (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346:245–250. doi:10.1038/346245a0
Koopman P, Gubbay J, Vivian N et al (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121. doi:10.1038/351117a0
Sinclair AH, Berta P, Palmer MS et al (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240–244. doi:10.1038/346240a0
Sekido R, Lovell-Badge R (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453:930–934. doi:10.1038/nature06944
Barrionuevo F, Bagheri-Fam S, Klattig J et al (2006) Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod 74:195–201. doi:10.1095/biolreprod.105.045930
Chaboissier M-C, Kobayashi A, Vidal VIP et al (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Dev Camb Engl 131:1891–1901. doi:10.1242/dev.01087
DeFalco T, Takahashi S, Capel B (2011) Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352:14–26. doi:10.1016/j.ydbio.2011.01.011
Yao HH-C, Whoriskey W, Capel B (2002) Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16:1433–1440. doi:10.1101/gad.981202
Barrios F, Filipponi D, Pellegrini M et al (2010) Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 123:871–880. doi:10.1242/jcs.057968
Bowles J, Feng C-W, Spiller C et al (2010) FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell 19:440–449. doi:10.1016/j.devcel.2010.08.010
Harley VR, Clarkson MJ, Argentaro A (2003) The molecular action and regulation of the testis-determining factors, SRY (sex-determining region on the Y chromosome) and SOX9 [SRY-related high-mobility group (HMG) box 9]. Endocr Rev 24:466–487
Foster JW, Dominguez-Steglich MA, Guioli S et al (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372:525–530. doi:10.1038/372525a0
Wagner T, Wirth J, Meyer J et al (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111–1120
Wright E, Hargrave MR, Christiansen J et al (1995) The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet 9:15–20. doi:10.1038/ng0195-15
Bi W, Deng JM, Zhang Z et al (1999) Sox9 is required for cartilage formation. Nat Genet 22:85–89. doi:10.1038/8792
Bi W, Huang W, Whitworth DJ et al (2001) Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A 98:6698–6703. doi:10.1073/pnas.111092198
Schafer AJ, Foster JW, Kwok C et al (1996) Campomelic dysplasia with XY sex reversal: diverse phenotypes resulting from mutations in a single gene. Ann N Y Acad Sci 785:137–149
Barrionuevo F, Georg I, Scherthan H et al (2009) Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol 327:301–312. doi:10.1016/j.ydbio.2008.12.011
O’Bryan MK, Takada S, Kennedy CL et al (2008) Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev Biol 316:359–370. doi:10.1016/j.ydbio.2008.01.042
Denny P, Swift S, Connor F, Ashworth A (1992) An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J 11:3705–3712
Connor F, Wright E, Denny P et al (1995) The Sry-related HMG box-containing gene Sox6 is expressed in the adult testis and developing nervous system of the mouse. Nucleic Acids Res 23:3365–3372
Lefebvre V (2010) The SoxD transcription factors–Sox5, Sox6, and Sox13–are key cell fate modulators. Int J Biochem Cell Biol 42:429–432. doi:10.1016/j.biocel.2009.07.016
Kiefer JC (2007) Back to basics: Sox genes. Dev Dyn 236:2356–2366. doi:10.1002/dvdy.21218
Akiyama H, Chaboissier M-C, Martin JF et al (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16:2813–2828. doi:10.1101/gad.1017802
Kwan KY, Lam MMS, Krsnik Z et al (2008) SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci U S A 105:16021–16026. doi:10.1073/pnas.0806791105
Lai T, Jabaudon D, Molyneaux BJ et al (2008) SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 57:232–247. doi:10.1016/j.neuron.2007.12.023
Lefebvre V, Li P, de Crombrugghe B (1998) A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J 17:5718–5733. doi:10.1093/emboj/17.19.5718
Kiselak EA, Shen X, Song J et al (2010) Transcriptional regulation of an axonemal central apparatus gene, sperm-associated antigen 6, by a SRY-related high mobility group transcription factor, S-SOX5. J Biol Chem 285:30496–30505. doi:10.1074/jbc.M110.121590
Ikeda T, Zhang J, Chano T et al (2002) Identification and characterization of the human long form of Sox5 (L-SOX5) gene. Gene 298:59–68
Hersh CP, Silverman EK, Gascon J et al (2011) SOX5 is a candidate gene for chronic obstructive pulmonary disease susceptibility and is necessary for lung development. Am J Respir Crit Care Med 183:1482–1489. doi:10.1164/rccm.201010-1751OC
Smits P, Li P, Mandel J et al (2001) The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell 1:277–290
Martinez-Morales PL, Quiroga AC, Barbas JA, Morales AV (2010) SOX5 controls cell cycle progression in neural progenitors by interfering with the WNT-beta-catenin pathway. EMBO Rep 11:466–472. doi:10.1038/embor.2010.61
Perez-Alcala S, Nieto MA, Barbas JA (2004) LSox5 regulates RhoB expression in the neural tube and promotes generation of the neural crest. Dev Camb Engl 131:4455–4465. doi:10.1242/dev.01329
Stolt CC, Schlierf A, Lommes P et al (2006) SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev Cell 11:697–709. doi:10.1016/j.devcel.2006.08.011
Melichar HJ, Narayan K, Der SD et al (2007) Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science 315:230–233. doi:10.1126/science.1135344
Hu G-X, Lian Q-Q, Ge R-S et al (2009) Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol Metab 20:139–145. doi:10.1016/j.tem.2008.12.001
Mendis-Handagama SM, Ariyaratne HB (2001) Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod 65:660–671
Benton L, Shan LX, Hardy MP (1995) Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol 53:61–68
Wang G, Hardy MP (2004) Development of leydig cells in the insulin-like growth factor-I (igf-I) knockout mouse: effects of igf-I replacement and gonadotropic stimulation. Biol Reprod 70:632–639. doi:10.1095/biolreprod.103.022590
Dong L, Jelinsky SA, Finger JN et al (2007) Gene expression during development of fetal and adult Leydig cells. Ann N Y Acad Sci 1120:16–35. doi:10.1196/annals.1411.016
Wang R-S, Yeh S, Tzeng C-R, Chang C (2009) Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 30:119–132. doi:10.1210/er.2008-0025
Bowles J, Schepers G, Koopman P (2000) Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol 227:239–255. doi:10.1006/dbio.2000.9883
Yamashita A, Ito M, Takamatsu N, Shiba T (2000) Characterization of Solt, a novel SoxLZ/Sox6 binding protein expressed in adult mouse testis. FEBS Lett 481:147–151
Takamatsu N, Kanda H, Tsuchiya I et al (1995) A gene that is related to SRY and is expressed in the testes encodes a leucine zipper-containing protein. Mol Cell Biol 15:3759–3766
Singh AP, Harada S, Mishina Y (2009) Downstream genes of Sox8 that would affect adult male fertility. Sex Dev Genet Mol Biol Evol Endocrinol Embryol Pathol Sex Determ Differ 3:16–25. doi:10.1159/000200078
Schepers G, Wilson M, Wilhelm D, Koopman P (2003) SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J Biol Chem 278:28101–28108. doi:10.1074/jbc.M304067200
Kanai Y, Kanai-Azuma M, Noce T et al (1996) Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J Cell Biol 133:667–681
Kent J, Wheatley SC, Andrews JE et al (1996) A male-specific role for SOX9 in vertebrate sex determination. Dev Camb Engl 122:2813–2822
Morais da Silva S, Hacker A, Harley V et al (1996) Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet 14:62–68. doi:10.1038/ng0996-62
Vidal VP, Chaboissier MC, de Rooij DG, Schedl A (2001) Sox9 induces testis development in XX transgenic mice. Nat Genet 28:216–217. doi:10.1038/90046
Hemendinger RA, Gores P, Blacksten L et al (2002) Identification of a specific Sertoli cell marker, Sox9, for use in transplantation. Cell Transplant 11:499–505
Bhandari RK, Haque MM, Skinner MK (2012) Global genome analysis of the downstream binding targets of testis determining factor SRY and SOX9. PLoS One 7:e43380. doi:10.1371/journal.pone.0043380
Ascoli M (1981) Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108:88–95
Martin LJ, Boucher N, Brousseau C, Tremblay JJ (2008) The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through cooperation with Ca2+/calmodulin-dependent protein kinase I. Mol Endocrinol Baltim Md 22:2021–2037. doi:10.1210/me.2007-0370
Tremblay JJ, Viger RS (2001) GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology 142:977–986
Martin LJ, Boucher N, El-Asmar B, Tremblay JJ (2009) cAMP-induced expression of the orphan nuclear receptor Nur77 in MA-10 Leydig cells involves a CaMKI pathway. J Androl 30:134–145. doi:10.2164/jandrol.108.006387
Schreiber E, Matthias P, Müller MM, Schaffner W (1989) Rapid detection of octamer binding proteins with “mini-extracts”, prepared from a small number of cells. Nucleic Acids Res 17:6419
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Martin LJ, Tremblay JJ (2005) The human 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase type 2 promoter is a novel target for the immediate early orphan nuclear receptor Nur77 in steroidogenic cells. Endocrinology 146:861–869. doi:10.1210/en.2004-0859
Huang W, Zhou X, Lefebvre V, de Crombrugghe B (2000) Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9’s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol 20:4149–4158
Kaur G, Delluc-Clavieres A, Poon IKH et al (2010) Calmodulin-dependent nuclear import of HMG-box family nuclear factors: importance of the role of SRY in sex reversal. Biochem J 430:39–48. doi:10.1042/BJ20091758
O’Shaughnessy PJ, Willerton L, Baker PJ (2002) Changes in Leydig cell gene expression during development in the mouse. Biol Reprod 66:966–975
Viger RS, Robaire B (1995) Steady state steroid 5 alpha-reductase messenger ribonucleic acid levels and immunocytochemical localization of the type 1 protein in the rat testis during postnatal development. Endocrinology 136:5409–5415. doi:10.1210/endo.136.12.7588289
Chang H, Gao F, Guillou F et al (2008) Wt1 negatively regulates beta-catenin signaling during testis development. Dev Camb Engl 135:1875–1885. doi:10.1242/dev.018572
Matheu A, Maraver A, Collado M et al (2009) Anti-aging activity of the Ink4/Arf locus. Aging Cell 8:152–161. doi:10.1111/j.1474-9726.2009.00458.x
McGee SR, Narayan P (2013) Precocious puberty and Leydig cell hyperplasia in male mice with a gain of function mutation in the LH receptor gene. Endocrinology 154:3900–3913. doi:10.1210/en.2012-2179
Ottolenghi C, Omari S, Garcia-Ortiz JE et al (2005) Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet 14:2053–2062. doi:10.1093/hmg/ddi210
Rezende NC, Lee M-Y, Monette S et al (2011) Rex1 (Zfp42) null mice show impaired testicular function, abnormal testis morphology, and aberrant gene expression. Dev Biol 356:370–382. doi:10.1016/j.ydbio.2011.05.664
Arango NA, Kobayashi A, Wang Y et al (2008) A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 75:1154–1162. doi:10.1002/mrd.20858
Churchman ML, Roig I, Jasin M et al (2011) Expression of arf tumor suppressor in spermatogonia facilitates meiotic progression in male germ cells. PLoS Genet 7:e1002157. doi:10.1371/journal.pgen.1002157
Ueda R, Yoshida K, Kawase T et al (2007) Preferential expression and frequent IgG responses of a tumor antigen, SOX5, in glioma patients. Int J Cancer 120:1704–1711. doi:10.1002/ijc.22472
Blaise R, Grober J, Rouet P et al (1999) Testis expression of hormone-sensitive lipase is conferred by a specific promoter that contains four regions binding testicular nuclear proteins. J Biol Chem 274:9327–9334
Budde LM, Wu C, Tilman C et al (2002) Regulation of IkappaBbeta expression in testis. Mol Biol Cell 13:4179–4194. doi:10.1091/mbc.01-07-0373
Moretti C, Mencacci C, Frajese GV et al (2002) Growth hormone-releasing hormone and pituitary adenylate cyclase-activating polypeptide in the reproductive system. Trends Endocrinol Metab 13:428–435
Wunderle VM, Critcher R, Ashworth A, Goodfellow PN (1996) Cloning and characterization of SOX5, a new member of the human SOX gene family. Genomics 36:354–358. doi:10.1006/geno.1996.0474
Xu W, Zhang S, Qiu W et al (2009) Spermatogenesis-related ring finger gene ZNF230 promoter: identification and functional analysis. Mol Biol Rep 36:1187–1193. doi:10.1007/s11033-008-9296-2
Kasimiotis H, Myers MA, Argentaro A et al (2000) Sex-determining region Y-related protein SOX13 is a diabetes autoantigen expressed in pancreatic islets. Diabetes 49:555–561
Roose J, Korver W, Oving E et al (1998) High expression of the HMG box factor sox-13 in arterial walls during embryonic development. Nucleic Acids Res 26:469–476
Kerr GE, Young JC, Horvay K et al (2014) Regulated Wnt/beta-catenin signaling sustains adult spermatogenesis in mice. Biol Reprod 90:3. doi:10.1095/biolreprod.112.105809
Jordan BK, Shen JH-C, Olaso R et al (2003) Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/beta-catenin synergy. Proc Natl Acad Sci U S A 100:10866–10871. doi:10.1073/pnas.1834480100
Yamashita A, Suzuki S, Fujitani K et al (1998) cDNA cloning of a novel rainbow trout SRY-type HMG box protein, rtSox23, and its functional analysis. Gene 209:193–200
Pevny LH, Lovell-Badge R (1997) Sox genes find their feet. Curr Opin Genet Dev 7:338–344
Dufau ML (1988) Endocrine regulation and communicating functions of the Leydig cell. Annu Rev Physiol 50:483–508. doi:10.1146/annurev.ph.50.030188.002411
Landry D, Paré A, Jean S, Martin LJ (2015) Adiponectin influences progesterone production from MA-10 Leydig cells in a dose-dependent manner. Endocrine 48:957–967. doi:10.1007/s12020-014-0456-y
De Santa Barbara P, Bonneaud N, Boizet B et al (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol 18:6653–6665
Ivell R (1997) Biology of the relaxin-like factor (RLF). Rev Reprod 2:133–138
Acknowledgments
We would like to thank Dr. Mario Ascoli for generously providing the MA-10 cell line used in this study. This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada (#386557-2012 to L.J.M.) and the New Brunswick Innovation Foundation (NBIF) (#IAR2012 and IAR2013-029 to L.J.M.).
Conflict of interest
The authors declare that there is no conflict of interest that would prejudice there impartiality.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mikella Daigle and Pauline Roumaud have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Daigle, M., Roumaud, P. & Martin, L.J. Expressions of Sox9, Sox5, and Sox13 transcription factors in mice testis during postnatal development. Mol Cell Biochem 407, 209–221 (2015). https://doi.org/10.1007/s11010-015-2470-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2470-7