Abstract
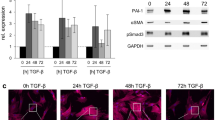
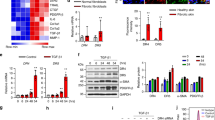
It is generally accepted that the apoptosis of myofibroblasts is a crucial event in the normal wound healing. Delay in myofibroblasts apoptosis results in fibrotic diseases such as systemic sclerosis (SSc). Transforming growth factor-β1 (TGF-β1) is an important cytokine to induce fibroblasts differentiation into myofibroblasts. Cellular Abelson (c-Abl) is known as a TGF-β1-modulating molecule in fibrosis. The role of c-Abl, TGF-β1, and their interaction in SSc myofibroblasts apoptosis has not yet been fully explored. The aim of this study was to evaluate whether TGF-β1 and inhibition of c-Abl influence Bax to Bcl-2 ratio and apoptosis in SSc and healthy dermal fibroblasts. We also would like to know whether there is interaction between TGF-β1 and c-Abl in connection with fibroblasts apoptosis or not. Bax to Bcl-2 ratio was determined using quantitative real-time polymerase chain reaction and immunoblotting. Apoptosis was detected using annexin V and nuclear staining with Hoechst dye. Our results demonstrated that inhibition of c-Abl increased SSc and healthy dermal fibroblasts susceptibility to apoptosis through increasing in Bax to Bcl-2 mRNA and protein ratios, whereas TGF-β1 promoted healthy fibroblasts resistance to apoptosis via decreasing Bax to Bcl-2 mRNA and protein ratios. In addition, c-Abl silencing reduced the effects of TGF-β1 on Bax to Bcl-2 mRNA and protein ratios. These results suggested that TGF-β1 and c-Abl individually may prevent the deletion of myofibroblasts from wounds and result in fibrosis. Results also proposed that silencing of c-Abl may promote myofibroblasts elimination from wound lesions through reduction in the TGF-β1 inhibitory effects on apoptosis.




Similar content being viewed by others
References
Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr, Carreira PE et al (2013) 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 72:1747–1755. doi:10.1136/annrheumdis-2013-204424
Bhattacharyya S, Wei J, Varga J (2012) Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol 8:42–54. doi:10.1038/nrrheum.2011.149
Varga J, Abraham D (2007) Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 117:557–567. doi:10.1172/jci31139
Darby IA, Hewitson TD (2007) Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 257:143–179. doi:10.1016/s0074-7696(07)57004-x
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200:500–503. doi:10.1002/path.1427
Verrecchia F, Mauviel A (2007) Transforming growth factor-beta and fibrosis. World J Gastroenterol 13:3056–3062
Pendergast AM (2002) The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res 85:51–100. doi:10.1016/S0065-230X(02)85003-5
Wilkes MC, Leof EB (2006) Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem 281:27846–27854. doi:10.1074/jbc.M603721200
Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB (2004) Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 114:1308–1316. doi:10.1172/jci19603
Wang S, Wilkes MC, Leof EB, Hirschberg R (2005) Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J 19:1–11. doi:10.1096/fj.04-2370com
Sun X, Majumder P, Shioya H, Wu F, Kumar S, Weichselbaum R, Kharbanda S, Kufe D (2000) Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem 275:17237–17240. doi:10.1074/jbc.C000099200
Iqbal S, Zhang S, Driss A, Liu ZR, Kim HR, Wang Y, Ritenour C, Zhau HE, Kucuk O, Chung LW et al (2012) PDGF upregulates Mcl-1 through activation of beta-catenin and HIF-1alpha-dependent signaling in human prostate cancer cells. PLoS ONE 7:e30764. doi:10.1371/journal.pone.0030764
Barisic K, Petrik J, Rumora L (2003) Biochemistry of apoptotic cell death. Acta Pharm 53:151–164
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, Rowell N, Wollheim F (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15:202–205
Takashima A (2001) Establishment of fibroblast cultures. Curr Protoc Cell Biol Chapter 2:Unit 2.1. doi:10.1002/0471143030.cb0201s00
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi:10.1038/nprot.2008.73
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Saile B, Matthes N, Knittel T, Ramadori G (1999) Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology 30:196–202. doi:10.1002/hep.510300144
Dybedal I, Guan F, Borge OJ, Veiby OP, Ramsfjell V, Nagata S, Jacobsen SE (1997) Transforming growth factor-beta1 abrogates Fas-induced growth suppression and apoptosis of murine bone marrow progenitor cells. Blood 90(9):3395–3403
Zhang HY, Phan SH (1999) Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am J Respir Cell Mol Biol 21:658–665. doi:10.1165/ajrcmb.21.6.3720
Sanchez A, Alvarez AM, Benito M, Fabregat I (1996) Apoptosis induced by transforming growth factor-beta in fetal hepatocyte primary cultures: involvement of reactive oxygen intermediates. J Biol Chem 271:7416–7422. doi:10.1074/jbc.271.13.7416
Yasuda K, Aoshiba K, Nagai A (2003) Transforming growth factor-beta promotes fibroblast apoptosis induced by H2O2. Exp Lung Res 29:123–134. doi:10.1080/01902140303773
Hagimoto N, Kuwano K, Inoshima I, Yoshimi M, Nakamura N, Fujita M, Maeyama T, Hara N (2002) TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol 168:6470–6478. doi:10.4049/jimmunol.168.12.6470
Brumatti G, Weinlich R, Chehab CF, Yon M, Amarante-Mendes GP (2003) Comparison of the anti-apoptotic effects of Bcr-Abl, Bcl-2 and Bcl-x(L) following diverse apoptogenic stimuli. FEBS Lett 541:57–63. doi:10.1016/S0014-5793(03)00299-0
Kumar S, Mishra N, Raina D, Saxena S, Kufe D (2003) Abrogation of the cell death response to oxidative stress by the c-Abl tyrosine kinase inhibitor STI571. Mol Pharmacol 63:276–282. doi:10.1124/mol.63.2.276
Lasfer M, Davenne L, Vadrot N, Alexia C, Sadji-Ouatas Z, Bringuier AF, Feldmann G, Pessayre D, Reyl-Desmars F (2006) Protein kinase PKC delta and c-Abl are required for mitochondrial apoptosis induction by genotoxic stress in the absence of p53, p73 and Fas receptor. FEBS Lett 580:2547–2552. doi:10.1016/j.febslet.2006.03.089
Distler JH, Jungel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, Michel BA, Hauser T, Schett G, Gay S et al (2007) Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum 56:311–322. doi:10.1002/art.22314
Akhmetshina A, Dees C, Pileckyte M, Maurer B, Axmann R, Jungel A, Zwerina J, Gay S, Schett G, Distler O et al (2008) Dual inhibition of c-Abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J 22:2214–2222. doi:10.1096/fj.07-105627
Karimizadeh E, Motamed N, Mahmoudi M, Jafarinejad-Farsangi S, Jamshidi A, Faridani H, Gharibdoost F (2015) Attenuation of fibrosis with selective inhibition of c-Abl by siRNA in systemic sclerosis dermal fibroblasts. Arch Dermatol Res 307:135–142. doi:10.1007/s00403-014-1532-0
Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC (2001) The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol 117:333–340. doi:10.1046/j.0022-202x.2001.01409.x
Acknowledgments
This research was supported by Iran National Science Foundation (INSF) (Grant No. 90001195) and Tehran university of Medical Sciences (Grant No. 90-03-41-13988).
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Karimizadeh, E., Gharibdoost, F., Motamed, N. et al. c-Abl silencing reduced the inhibitory effects of TGF-β1 on apoptosis in systemic sclerosis dermal fibroblasts. Mol Cell Biochem 405, 169–176 (2015). https://doi.org/10.1007/s11010-015-2408-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2408-0