Abstract
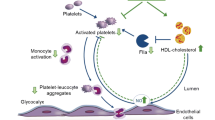
This study was designed to assess the potential effect of vitamin D3 (VD3) in avoiding atherothrombosis by modulation of lipid metabolism and platelet activation in type 1 diabetic rats. Male wistar rats were divided into eight groups (n = 5–10): Control/Saline (Sal); Control/Metformin 500 mg/kg (Metf); Control/Vitamin D3 90 µg/kg (VD3); Control/Metformin 500 mg/kg + VD3 90 µg/kg (Metf + VD3); Diabetic/Saline (Sal); Diabetic/Metformin 500 mg/kg (Metf); Diabetic/Vitamin D3 90 µg/kg (VD3); Diabetic/Metformin 500 mg/kg + VD3 90 µg/kg (Metf + VD3). Treatments were administered during 30 days after diabetes induction with streptozotocin (STZ). After 31 days, the rats were euthanized and blood was collected and separated into serum and platelets, both used for lipid profile and ectonucleotidase activity assays, respectively. Ectonucleoside triphosphate phosphohydrolase (E-NTPDase), ectonucleotide pyrophosphatase/phosphodiesterase (E-NPP), and 5′-nucleotidase and adenosine deaminase (E-ADA) were significantly higher in the Diabetic than in Control group. Treatment with Metf and/or VD3 prevented the increase in NTPDase and E-NPP activities in diabetic rats. Only Metf + VD3 significantly prevented the increase in 5′-nucleotidase. VD3 alone, but not Metf, prevented the increase in ADA activity when compared to saline-treated diabetic rats. Treatment of rats with VD3, Metf, and Metf + VD3 was also effective in the prevention of lipid metabolism disorder in diabetic and was able to ameliorate lipid metabolism in non-diabetic rats. These results provide evidence for the potential of Metf and VD3 in the treatment of platelet dysfunction and lipid metabolism impairment in T1D, which may be important in the control and prevention of atherothrombosis in diabetes.



Similar content being viewed by others
Abbreviations
- 25(OH)D3 :
-
25-Hydroxyvitamin D3
- T1D:
-
Type 1 Diabetes
- VD3 :
-
Vitamin D3
- Metf:
-
Metformin
- Metf + VD3 :
-
Metformin plus Vitamin D3
- CVD:
-
Cardiovascular Disease
- TG:
-
Triglycerides
- LDL-c:
-
Low Density Lipid Cholesterol
- VLDL-c:
-
Very Low Density Lipid Cholesterol
- HDL-c:
-
High Density Lipid Cholesterol
- VDR:
-
Vitamin D Receptor
- PGI2:
-
Prostacyclin
- ADA:
-
Adenosine desaminase
- NTPDase:
-
Ectonucleoside Triphosphate Phosphoydrolase
- E-NPP:
-
Ectonucleotide pyrophosphatase/phosphodiesterase
- Sal:
-
Saline
- STZ:
-
Streptozotocin
References
Lambert P, Bingley PJ (2002) What is type 1 diabetes? Medicine 30:1–5. doi:10.1383/medc.30.1.1.28264
Junod A, Lambert AE, Stauffacher W et al (1969) Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest 48:2129–2139. doi:10.1172/JCI106180
Gawaz M, Langer H, May AE (2005) Platelets in inflammation and atherogenesis. J Clin Invest 115:3378–3384. doi:10.1172/JCI27196
Birk AV, Broekman MJ, Gladek EM et al (2002) Role of extracellular ATP metabolism in regulation of platelet reactivity. J Lab Clin Med 140:166–175
Lunkes GI, Lunkes DS, Morsch VM et al (2004) NTPDase and 5′-nucleotidase activities in rats with alloxan-induced diabetes. Diabetes Res Clin Pract 65:1–6. doi:10.1016/j.diabres.2003.11.016S0168822703003164
Lunkes GI, Lunkes DS, Leal D et al (2008) Effect of high glucose levels in human platelet NTPDase and 5′-nucleotidase activities. Diabetes Res Clin Pract 81:351–357. doi:10.1016/j.diabres.2008.06.001
Lunkes GI, Lunkes D, Stefanello F et al (2003) Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res 109:189–194
Schmatz R, Schetinger MR, Spanevello RM et al (2009) Effects of resveratrol on nucleotide degrading enzymes in streptozotocin-induced diabetic rats. Life Sci 84:345–350. doi:10.1016/j.lfs.2008.12.019
Berthezène F (2002) Diabetic dyslipidaemia. Br J Diabetes Vasc Dis 2:S12–S17
Gargiulo P, Caccese D, Pignatelli P et al (2002) Metformin decreases platelet superoxide anion production in diabetic patients. Diabetes Metab Res Rev 18:156–159. doi:10.1002/dmrr.282
Chan NN (2001) Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol 38:2131–2132
El-Batran SA, Abdel-Salam OM, Nofal SM et al (2006) Effect of rosiglitazone and nateglinide on serum glucose and lipid profile alone or in combination with the biguanide metformin in diabetic rats. Pharmacol Res 53:69–74. doi:10.1016/j.phrs.2005.08.008
Ghatak SB, Dhamecha PS, Bhadada SV et al (2011) Investigation of the potential effects of metformin on atherothrombotic risk factors in hyperlipidemic rats. Eur J Pharmacol 659:213–223. doi:10.1016/j.ejphar.2011.03.029
Kassi E, Adamopoulos C, Basdra EK et al (2013) Role of vitamin D in atherosclerosis. Circulation 128:2517–2531. doi:10.1161/CIRCULATIONAHA.113.002654
Peeyush KT, Savitha B, Sherin A et al (2010) Cholinergic, dopaminergic and insulin receptors gene expression in the cerebellum of streptozotocin-induced diabetic rats: functional regulation with Vitamin D3 supplementation. Pharmacol Biochem Behav 95:216–222. doi:10.1016/j.pbb.2010.01.008
George N, Kumar TP, Antony S et al (2012) Effect of vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic rats. Br J Nutr 108:1410–1418. doi:10.1017/S0007114511006830
Rudnicki PM, Molsted-Pedersen L (1997) Effect of 1,25-dihydroxycholecalciferol on glucose metabolism in gestational diabetes mellitus. Diabetologia 40:40–44. doi:10.1007/s001250050640
Borissova AM, Tankova T, Kirilov G et al (2003) The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract 57:258–261
Clark SA, Stumpf WE, Sar M (1981) Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes 30:382–386
Chiu KC, Chu A, Go VL et al (2004) Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 79:820–825
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157:375–380
Furstenau CR, Trentin Dda S, Barreto-Chaves ML et al (2006) Ecto-nucleotide pyrophosphatase/phosphodiesterase as part of a multiple system for nucleotide hydrolysis by platelets from rats: kinetic characterization and biochemical properties. Platelets 17:84–91. doi:10.1080/09537100500246641
Guisti G, Galanti B (1984) Colorimetric method. Bergmeyer HU. Verlag Chemie, Weinheim, pp 315–323
Bachorik PS, Albers JJ (1986) Precipitation methods for quantification of lipoproteins. Methods Enzymol 129:78–100
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Calgaroto NS, Thome GR, da Costa P et al (2014) Effect of vitamin D3 on behavioural and biochemical parameters in diabetes type 1-induced rats. Cell Biochem Funct 32:502–510. doi:10.1002/cbf.3044
Del Pino-Montes J, Benito GE, Fernandez-Salazar MP et al (2004) Calcitriol improves streptozotocin-induced diabetes and recovers bone mineral density in diabetic rats. Calcif Tissue Int 75:526–532. doi:10.1007/s00223-004-0118-9
Carr ME (2001) Diabetes mellitus: a hypercoagulable state. J Diabetes Complicat 15:44–54
Bours MJ, Swennen EL, Di Virgilio F et al (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112:358–404. doi:10.1016/j.pharmthera.2005.04.013
Rutkiewicz J, Gorski J (1990) On the role of insulin in regulation of adenosine deaminase activity in rat tissues. FEBS Lett 271:79–80
Kurtul N, Pence S, Akarsu E et al (2004) Adenosine deaminase activity in the serum of type 2 diabetic patients. Acta Medica (Hradec Kralove) 47:33–35
Matsuno H, Tokuda H, Ishisaki A et al (2005) P2Y12 receptors play a significant role in the development of platelet microaggregation in patients with diabetes. J Clin Endocrinol Metab 90:920–927. doi:10.1210/jc.2004-0137
Soslau G, McKenzie RJ, Brodsky I et al (1995) Extracellular ATP inhibits agonist-induced mobilization of internal calcium in human platelets. Biochim Biophys Acta 1268:73–80
Soslau G, Youngprapakorn D (1997) A possible dual physiological role of extracellular ATP in the modulation of platelet aggregation. Biochim Biophys Acta 1355:131–140
Bodin P, Burnstock G (1996) ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol 27:872–875
Redegeld FA, Caldwell CC, Sitkovsky MV (1999) Ecto-protein kinases: ecto-domain phosphorylation as a novel target for pharmacological manipulation? Trends Pharmacol Sci 20:453–459
Furstenau CR, Spier AP, Rucker B et al (2004) The effect of ebselen on adenine nucleotide hydrolysis by platelets from adult rats. Chem Biol Interact 148:93–99. doi:10.1016/j.cbi.2004.04.003
Mamputu JC, Wiernsperger NF, Renier G (2003) Antiatherogenic properties of metformin: the experimental evidence. Diabetes Metab 29:6S71–6S76
Winocour PD, Laimins M, Colwell JA (1984) Platelet survival in streptozotocin-induced diabetic rats. Thromb Haemost 51:307–312
Martin FJ, Miguez JM, Aldegunde M et al (1995) Platelet serotonin transport is altered in streptozotocin-induced diabetic rats. Life Sci 56:1807–1815
James J, Padayatti P, Paul T et al (1997) Platelet monoamine changes in diabetic patients and streptozotocin-induced diabetic rats. Curr Sci 72:137–139
Paton RC (1979) Platelet survival in diabetes mellitus using an aspirin-labelling technique. Thromb Res 15:793–802
Leytin V, Freedman J (2003) Platelet apoptosis in stored platelet concentrates and other models. Transfus Apher Sci 28:285–295. doi:10.1016/S1473-0502(03)00048-X
Pilo R, Aharony D, Raz A (1981) Testosterone potentiation of ionophore and ADP induced platelet aggregation: relationship to arachidonic acid metabolism. Thromb Haemost 46:538–542
Khetawat G, Faraday N, Nealen ML et al (2000) Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood 95:2289–2296
Bockow B, Kaplan TB (2013) Refractory immune thrombocytopenia successfully treated with high-dose vitamin D supplementation and hydroxychloroquine: two case reports. J Med Case Rep. doi:10.1186/1752-1947-7-91
Silvagno F, De Vivo E, Attanasio A et al (2010) Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS One 5:e8670. doi:10.1371/journal.pone.0008670
Song LN, Cheng T (1993) Glucocorticoid-induced growth inhibition and differentiation of a human megakaryoblastic leukemia cell line: involvement of glucocorticoid receptor. Stem Cells 11:312–318. doi:10.1002/stem.5530110409
Michno A, Bielarczyk H, Pawelczyk T et al (2007) Alterations of adenine nucleotide metabolism and function of blood platelets in patients with diabetes. Diabetes 56:462–467. doi:10.2337/db06-0390
Grundy SM (2006) Diabetes and coronary risk equivalency: what does it mean? Diabetes Care 29:457–460
Chahil TJ, Ginsberg HN (2006) Diabetic dyslipidemia. Endocrinol Metab Clin North Am 35(491–510):vii–viii. doi:10.1016/j.ecl.2006.06.002
Jaiswal M, Schinske A, Pop-Busui R (2014) Lipids and lipid management in diabetes. Best Pract Res Clin Endocrinol Metab 28:325–338. doi:10.1016/j.beem.2013.12.001
Maahs DM, Dabelea D, D’Agostino RB Jr et al (2013) Glucose control predicts 2-year change in lipid profile in youth with type 1 diabetes. J Pediatr 162(101–107):e101. doi:10.1016/j.jpeds.2012.06.006
Valdivielso P, Sanchez-Chaparro MA, Calvo-Bonacho E et al (2009) Association of moderate and severe hypertriglyceridemia with obesity, diabetes mellitus and vascular disease in the Spanish working population: results of the ICARIA study. Atherosclerosis 207:573–578. doi:10.1016/j.atherosclerosis.2009.05.024
Hamzah RU, Odetola AA, Erukainure OL et al (2012) Peperomia pellucida in diets modulates hyperglyceamia, oxidative stress and dyslipidemia in diabetic rats. J Acute Dis 1(2):135–140
Gonçalves AESS, Lellis-Santos C, Curi R et al (2014) Frozen pulp extracts of camu-camu(Myrciaria dubia McVaugh) attenuate the hyperlipidemia and lipid peroxidation of Type 1 diabetic rats. Food Res Int 64:1–8
Pillai SI, Subramanian SP, Kandaswamy M (2014) Antidyslipidemic effect of a novel vanadium-3-hydroxy flavone complex in streptozotocin-induced experimental diabetes in rats. Biomed Prev Nut 4:189–193
Ugochukwu NH, Figgers CL (2007) Attenuation of plasma dyslipidemia and oxidative damage by dietary caloric restriction in streptozotocin-induced diabetic rats. Chem Biol Interact 169:32–41. doi:10.1016/j.cbi.2007.05.002
Conflict of interests
The authors declare that they have no Conflict of interests.
Compliance with ethical standards
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Federal University of Santa Maria (protocol under number: 23/2012). All efforts were made to minimize suffering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calgaroto, N.S., da Costa, P., Cardoso, A.M. et al. Vitamin D3 prevents the increase in ectonucleotidase activities and ameliorates lipid profile in type 1 diabetic rats. Mol Cell Biochem 405, 11–21 (2015). https://doi.org/10.1007/s11010-015-2390-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2390-6