Abstract
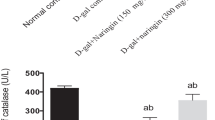
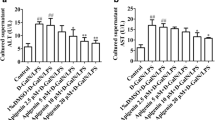
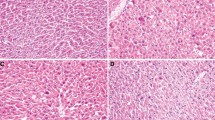
FGF21 is recently discovered with pleiotropic effects on glucose and lipid metabolism. However, the potential protective effect of FGF21 against d-gal-induced injury in the liver has not been demonstrated. The aim of this study is to investigate the pathophysiological role of FGF21 on hepatic oxidative injury and apoptosis in mice induced by d-gal. The 3-month-old Kunming mice were subcutaneously injected with d-gal (180 mg kg−1 d−1) for 8 weeks and administered simultaneously with FGF21 (5 or 1 mg kg−1 d−1). Our results showed that the administration of FGF21 significantly alleviated histological lesion including structure damage, degeneration, and necrosis of hepatocytes induced by d-gal, and attenuated the elevation of liver injury markers, serum AST, and ALP in a dose-dependent manner. FGF21 treatment also suppressed d-gal-induced profound elevation of ROS production and oxidative stress, as evidenced by an increase of the MDA level and depletion of the intracellular GSH level in the liver, and restored the activities of antioxidant enzymes SOD, CAT, GSH-Px, and T-AOC. Moreover, FGF21 treatment increased the nuclear abundance of Nrf2 and subsequent up regulation of several antioxidant genes. Furthermore, a TUNEL assay showed that d-gal-induced apoptosis in the mouse liver was significantly inhibited by FGF21. The expression of caspase-3 was markedly inhibited by the treatment of FGF21 in the liver of d-gal-treated mice. The levels of PI3K and PBK/Akt were also largely enhanced, which in turn inactivated pro-apoptotic signaling events, restoring the balance between pro- and anti-apoptotic Bcl-2 and Bax proteins in the liver of d-gal-treated mice. In conclusion, these results suggest that FGF21 protects the mouse liver against d-gal-induced hepatocyte oxidative stress via enhancing Nrf2-mediated antioxidant capacity and apoptosis via activating PI3K/Akt pathway.







Similar content being viewed by others
Abbreviations
- FGF21:
-
Fibroblast growth factor 21
- d-Gal:
-
d-Galactose
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GSH-Px:
-
Glutathione peroxidase
- T-AOC:
-
Total antioxidation capability
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- TUNEL:
-
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- PI3K:
-
Phosphoinositide-3-kinase
- PBK/Akt:
-
Protein kinase B
References
Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W, Liu J (2004) d-Galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology 5:317–325
Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q (2007) Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem Pharmacol 74:1078–1090
Ho SC, Liu JH, Wu RY (2003) Establishment of the mimetic aging effect in mice caused by d-galactose. Biogerontology 4:15–18
Ramana BV, Kumar VV, Krishna PN, Kumar CS, Reddy PU, Raju TN (2006) Effect of quercetin on galactose-induced hyperglycaemic oxidative stress in hepatic and neuronal tissues of Wistar rats. Acta Diabetol 43:135–141
Long JG, Wang XM, Gao HX, Liu Z, Liu CS, Miao MY, Cui X, Packer L, Liu JK (2007) d-Galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology 8:373–381
Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29:323–333
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Jacob MH, Janner Dda R, Araújo AS, Jahn MP, Kucharski LC, Moraes TB, Dutra Filho CS, Ribeiro MF, Belló-Klein A (2010) Redox imbalance influence in the myocardial Akt activation in aged rats treated with DHEA. Exp Gerontol 45:957–963
Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46:443–453
Cho SG, Choi EJ (2002) Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol 35:24–27
Porta C, Figlin RA (2009) Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol 182:2569–2577
Liu CM, Ma JQ, Sun YZ (2012) Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol 258:330–342
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB (2005) FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635
Ryden M (2009) Fibroblast growth factor 21: an overview from a clinical perspective. Cell Mol Life Sci 66:2067–2073
Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J (2006) Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55:2470–2478
Feingold KR, Grunfeld C, Heuer JG, Gupta A, Cramer M, Zhang T, Shigenaga JK, Patzek SM, Chan ZW, Moser A, Bina H, Kharitonenkov A (2012) FGF21 is increased by inflammatory stimuli and protects leptin-deficient ob/ob mice from the toxicity of sepsis. Endocrinology 153:2689–2700
Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F (2013) Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun 4:2019
Cong WT, Ling J, Tian HS, Ling R, Wang Y, Huang BB, Zhao T, Duan YM, Jin LT, Li XK (2013) Proteomic study on the protective mechanism of fibroblast growth factor 21 to ischemia–reperfusion injury. Can J Physiol Pharmacol 91:973–984
Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC (2009) FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106:10853–10858
Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA (2007) Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425
Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA (2008) Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8:77–83
Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, Wang Y, Lam KS, Xu A (2014) FGF21 Protects against acetaminophen-induced hepatotoxicity by potentiating PGC-1α-mediated antioxidant capacity in mice. Hepatology. doi:10.1002/hep.27060
Guicciardi ME, Gores GJ (2005) Apoptosis: a mechanism of acute and chronic liver injury. Gut 54:1024–1033
Ruan Q, Liu F, Gao Z, Kong D, Hu X, Shi D, Bao Z, Yu Z (2013) The anti-inflamm-aging and hepato protective effects of huperzine A in d-galactose-treated rats. Mech Ageing Dev 134:89–97
Zhang ZF, Lu J, Zheng YL, Hu B, Fan SH, Wu DM, Zheng ZH, Shan Q, Liu CM (2010) Purple sweet potato color protects mouse liver against d-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing PI3K/Akt pathway. Food Chem Toxicol 48:2500–2507
Rana SV (2008) Metals and apoptosis: recent developments. J Trace Elem Med Biol 22:262–284
Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: ménage à trois. Mutat Res 674:3–22
Lawen A (2003) Apoptosis—an introduction. BioEssays 25:888–896
Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J, Dimri GP (2003) Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol 2:389–401
Satyanarayana A, Wiemann SU, Buer J, Lauber J, Dittmar KE, Wustefeld T, Blasco MA, Manns MP, Rudolph KL (2003) Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. EMBO J 22:4003–4013
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B (2009) Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food Chem Toxicol 47:496–501
Chen HL, Wang CH, Kuo YW, Tsai CH (2011) Antioxidative and hepatoprotective effects of fructo-oligosaccharide in d-galactose-treated Balb/cJ mice. Br J Nutr 105:805–809
Zhang C, Shao M, Yang H, Chen L, Yu L, Cong W, Tian H, Zhang F, Cheng P, Jin L, Tan Y, Li X, Cai L, Lu X (2013) Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS One 8:e82275
Sun Y (1990) Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 8:583–599
Kaspar JW, Niture SK, Jaiswal AK (2009) Nrf 2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med 47:1304–1309
Li YN, Guo Y, Xi MM, Yang P, Zhou XY, Yin S, Hai CX, Li JG, Qi XJ (2014) Saponins from Aralia taibaiensis attenuate d-galactose-induced aging in rats by activating FOXO3a and Nrf2 pathways. Oxid Med Cell Longev. doi:10.1155/2014/320513
Wu JH, Batist G (2013) Glutathione and glutathione analogues, therapeutic potentials. Biochim Biophys Acta 1830:3350–3353
Han YH, Kim SZ, Kim SH, Park WH (2008) Arsenic trioxide inhibits the growth of Calu-6 cells via inducing a G2 arrest of the cell cycle and apoptosis accompanied with the depletion of GSH. Cancer Lett 18:40–55
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C (2003) PI3K/Akt and apoptosis: size matters. Oncogene 22:8983–8998
Yin F, Liu J, Zheng X, Guo L, Xiao H (2010) Geniposide induces the expression of heme oxygenase-1 via PI3K/Nrf2-signaling to enhance the antioxidant capacity in primary hippocampal neurons. Biol Pharm Bull 33:1841–1846
Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275:10761–10766
Higuchi M, Honda T, Proske RJ, Yeh ET (1998) Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 17:2753–2760
Acknowledgments
Yinhang Yu, Fuliang Bai, Wenfei Wang, and Deshan Li conceived and designed the experiments. Yinhang Yu, Yaonan Liu, Yongbi Yang, Qingyan Yuan, Dehua Zou, Tong Zhang, Siming Li, Susu Qu, Guiyou Tian, and YunYe Liu performed the experiments. Yin hang Yu, Guiping Ren, and Deshan Li analyzed the data. Yinhang Yu wrote the paper. Deshan Li revised the paper.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yinhang Yu and Fuliang Bai have contributed equally to this study and are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Y., Bai, F., Liu, Y. et al. Fibroblast growth factor (FGF21) protects mouse liver against d-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol Cell Biochem 403, 287–299 (2015). https://doi.org/10.1007/s11010-015-2358-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2358-6