Abstract
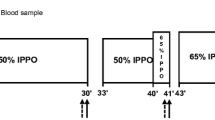
As the demand for hepatic glucose production increases during exercise, regulation of liver substrate choice and gluconeogenic activity becomes essential. The aim of the present study was to investigate the effect of a single exercise bout on gluconeogenic protein content and regulation of enzymes involved in substrate utilization in the liver. Mice were subjected to 1 h of treadmill exercise, and livers were removed immediately, 4 or 10 h after exercise. Glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxylase (PEPCK) mRNA contents in the liver increased immediately after exercise, while the PEPCK protein content increased at 10 h of recovery. Furthermore, 5′AMP-activated protein kinase (AMPK), acetyl-CoA carboxylase (ACC), and pyruvate dehydrogenase (PDH)-E1α Ser293 phosphorylations decreased immediately after exercise. In addition, PDH kinase 4 (PDK4) mRNA and protein content increased immediately after exercise and at 10 h of recovery, respectively. These findings suggest that acute changes in PEPCK and G6Pase protein contents do not contribute to the regulation of gluconeogenic enzyme activity during 1 h of non-exhaustive exercise. In addition, the observation that PDH-E1α, AMPK, and ACC phosphorylation decreased immediately after exercise may indicate that carbohydrates rather than fatty acids are utilized for oxidation in the liver during non-exhaustive exercise.






Similar content being viewed by others
References
Ahlborg G et al (1974) Substrate Turnover during prolonged exercise in man: splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest 53(4):1080–1090
Wasserman DH (1995) Regulation of glucose fluxes during exercise in the postabsorptive state. Ann Rev Physiol 57(1):191–218
Haase TN et al (2011) Role of PGC-1α in exercise and fasting-induced adaptations in mouse liver. Am J Physiol Regul Integr Comp Physiol 301(5):R1501–R1509
Friedman JE (1994) Role of glucocorticoids in activation of hepatic PEPCK gene transcription during exercise. Am J Physiol 266(4):e560–e566
Banzet S et al (2009) Control of gluconeogenic genes during intense/prolonged exercise: hormone-independent effect of muscle-derived IL-6 on hepatic tissue and PEPCK mRNA. J Appl Physiol 107(6):1830–1839
Fritsche L et al (2010) IL-6 deficiency in mice neither impairs induction of metabolic genes in the liver nor affects blood glucose levels during fasting and moderately intense exercise. Diabetologia 53(8):1732–1742
Hoene M et al (2009) Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J Physiol 587(1):241–252
Park H et al (2002) Coordinate regulation of Malonyl-CoA Decarboxylase, sn-Glycerol-3-phosphate Acyltransferase, and Acetyl-CoA Carboxylase by AMP-activated Protein Kinase in rat tissues in response to exercise. J Bio Chem 277(36):32571–32577
Kelly M et al (2004) AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 320(2):449–454
Berglund ED et al (2010) Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARα and FGF21 transcripts in vivo. Am J Physiol—Endocrinol Metabol 299(4):E607–E614
Carlson CL, Winder WW (1999) Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. J Appl Physiol 86(2):669–674
Cori CF, Cori GT (1928) Glycogen formation in the liver from d- and l-lactic acid. J Bio Chem 81(2):14
Dohm GL, Newsholme EA (1983) Metabolic control of hepatic gluconeogenesis during exercise. Biochem J 212(3):633–639
Randle PJ (1986) Fuel selection in animals. Biochem Soc Trans 14:799–806
Nam Ho J, Robert AH (2008) Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol—Endocrinol Metabol 295(1):E46–E54
Brandt C et al (2012) IL-6 regulates exercise and training-induced adaptations in subcutaneous adipose tissue in mice. Acta Physiologica 205(2):224–235
Adser, H., et al. (2011). IL-6 modifies mRNA expression in mouse. Acta Physiologica, p. no–no.
Pedersen, L., et al. (2011). Exercise-induced liver CXCL-1 expression is linked to muscle derived IL-6 expression. The Journal of Physiology.
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol–chloroform extraction. Anal Biochem 162(1):156–159
Pilegaard H et al (2000) Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. AJP 279(4):E806–E814
Lundby C et al (2005) Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol 95(4):351–360
Birk JB, Wojtaszewski JFP (2006) Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577(3):1021–1032
Castaño JG, Nieto A, Felíu JE (1979) Inactivation of phosphofructokinase by glucagon in rat hepatocytes. J Bio Chem 254(13):5576–5579
Dohm GL, Kasperek GJ, Barakat HA (1985) Time course of changes in gluconeogenic enzyme activities during exercise and recovery. Am J Physiol 249(1):E6–E11
Korotchkina LG, Patel MS (1995) Mutagenesis Studies of the Phosphorylation Sites of Recombinant Human Pyruvate Dehydrogenase. Site-specific regulation. J Bio Chem 270(24):14297–14304
Yi X et al (2013) Effects of acute exercise and chronic exercise on the liver Leptin-AMPK-ACC signaling pathway in rats with Type 2 diabetes. J Diabetes Res 2013:9
Kiilerich K et al (2010) Low muscle glycogen and elevated plasma free fatty acid modify but do not prevent exercise-induced pdh activation in human skeletal muscle. Diabetes 59(1):26–32
Dyar KA et al (2014) Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab 3(1):29–41
Acknowledgments
The study was supported by grants from the Novo Nordisk Foundation, the Lundbeck Foundation, The Danish Research Foundation, The Danish Council for Independent Research in the Natural Sciences, and the Augustinus Foundation.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knudsen, J.G., Biensø, R.S., Hassing, H.A. et al. Exercise-induced regulation of key factors in substrate choice and gluconeogenesis in mouse liver. Mol Cell Biochem 403, 209–217 (2015). https://doi.org/10.1007/s11010-015-2351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2351-0